Introduction of Heat-Stable Carbetocin for Postpartum Hemorrhage Prevention in Public Sector Hospitals in Kenya: Provider Experience and Policy Insights

* Corresponding author: Daisy Ruto, Project Director, Smiles for Mothers, Kenya, Nairobi. Tel: +254 70 882 2611 Daisy.Ruto@jhpiego.org
-
Received: ,
Accepted: ,
How to cite this article: Ruto D, Muthamia M, Njeri E, Nyaga F, Muia C, Kiio M, et al. Introduction of heat-stable carbetocin for postpartum hemorrhage prevention in public sector hospitals in Kenya: Provider experience and policy insights. Int J MCH AIDS. 2024;13:S28-37. doi: 10.25259/IJMA_4_2024
Abstract
Background and Objective
In Kenya, the leading cause of maternal deaths is obstetric hemorrhage (39.5%), with postpartum hemorrhage (PPH) accounting for 50% with quality of uterotonics as one of the biggest challenges. The World Health Organization (WHO) in 2018 included heat-stable carbetocin (HSC) for the prevention of PPH in settings where the quality of oxytocin cannot be guaranteed. Maintenance of the cold chain for uterotonics is a challenge. HSC does not require refrigeration, reducing pressure on the fragile cold chain infrastructure. The main objective was to understand PPH prevention knowledge, experience, and perspectives, including uterotonic use, by policymakers and healthcare providers (HCPs) in the public health sector in ten counties in Kenya. HCP knowledge, perception, and experience were assessed after the HSC introduction.
Methods
The mixed methods study was implemented in 39 secondary and tertiary public hospitals from ten counties. Quantitative interviews targeting 171 HCPs at baseline and end-line were collected using REDCap software (v5.26.4) and analyzed using Stata version 17. Qualitative data was collected from 19 policymakers at the national, county, sub county, and health facility levels and analyzed using NVIVO 12.
Results
At the end line, 98.8% had administered HSC for the prevention of PPH, while 96.5% of the HCPs were aware that their facilities had protocols/guidelines in place on the use of HSC. To enhance awareness of WHO recommendations on the use of HSC among HCPs, a top-down approach was used. Over 90% of HCPs agreed that HSC was easy to administer and distinguish from other uterotonics. Policymakers agreed that there was value in the HSC introduction in the public health sector that experiences cold chain challenges and recommended budgetary allocation.
Conclusion and Global Health Implications
The findings demonstrate that HCP’s knowledge, perception, and experience coupled with the policymaker’s perspective is the key to the introduction of HSC in the public sector. Policymakers find value in introducing HSC as it alleviates challenges with the fragile cold chain systems. This study contributes to the global body of knowledge on the introduction of lifesaving commodities, which is anticipated to potentially improve PPH prevention and management, and hence reduce maternal mortality.
Keywords
HSC
Postpartum Hemorrhage
Uterotonics
World Health Organization
PPH Recommendations
INTRODUCTION
Background of the Study
In 2018, the World Health Organization (WHO) recommended Active Management of the Third Stage of Labor (AMTSL) to prevent postpartum hemorrhage (PPH),[1] defined as blood loss of 500 ml within 24 hours after birth. Globally, PPH is the leading cause of maternal mortality and accounts for 30% in developing countries. Almost all maternal deaths (94%) occur in low-income and lower-middle-income countries (LMICs), and almost two-thirds (65%) occur in the African region with more than two-thirds due to PPH.[1] The leading cause of maternal deaths in Kenya is obstetric hemorrhage (39.5%), with 50% being attributed to PPH. Kenya is working toward reducing maternal deaths to less than 70 maternal deaths per 100,000 live births by 2030.[2]
Oxytocin has been used as the first line uterotonic for PPH prevention and treatment. It requires cold chain transport and storage and these requirements are not universally available in all settings where women give birth and specifically pose a challenge in low-and-middle-income countries (LMICs).
WHO updated its PPH prevention guideline in 2018 to include HSC for the prevention of excessive bleeding in settings where oxytocin is unavailable or its quality cannot be guaranteed and where its cost is comparable to other effective uterotonics.[3] HSC does not require refrigeration and has a longer half-life and duration of action in comparison to oxytocin, and the safety profiles of the two drugs are comparable.[4] Global efforts with the manufacturer allowed HSC to be provided in the public sector of LMICs at a comparable price to that of oxytocin.[5] The Ministry of Health (MOH), Kenya, revised the National Guidelines on Quality Obstetrics and Perinatal Care in February 2022 to allow the use of HSC for the prevention of PPH.
Training of healthcare providers (HCPs) on evidence-based recommendations strengthens the standardization of PPH services. However, clinical guidelines do not always translate to practice.[6] In Kenya, a study found low provider knowledge of the signs of PPH (43%) less than 1% of HCPs had complete knowledge of PPH care provision, and only 50% of women received AMTSL according to standards.[7] In a study conducted in Nigeria, only 46% of respondents (52.8% in private and 40.0% in the public sector) had proper knowledge of oxytocin storage and cold chain maintenance.[8]
In addition to low provider knowledge and skills, there is a myriad of challenges facing PPH care in Kenya, including the often compromised cold chains for heat-sensitive uterotonics, poor forecasting and quantification, and lack of PPH awareness at the community level.[9] In a recent systemic review, overall 48.9% of 1,890 uterotonic samples (from 19 studies) failed quality tests (about 40% each for oxytocin and misoprostol).[10] Hence, a significant barrier to reducing maternal mortality and severe morbidity due to PPH is due to a lack of quality medicines, particularly in limited-resource settings.[11]
The study was implemented in 39 secondary and tertiary public hospitals from ten counties. The intervention package included: (i) HCPs facility-based training, post-training follow-up, mentorship, and PPH drills; (ii) dissemination of national obstetric and perinatal care guidelines, PPH protocols; (iii) dissemination of client literacy materials; (iv) quality improvement activities, including facility review meetings, data quality improvement, including Maternal Perinatal Death Surveillance and Response (MPDSR); and (v) distribution and utilization of HSC in 39 public health facilities. This manuscript summarizes experiences from the introduction of HSC in the public health sector.
Objectives of the Study
The Smiles for Mothers (SfM) Project was funded by Merck Sharp & Dohme (MSD) for Mothers to strengthen the MOH’s response to PPH prevention and management through innovative health system improvements. SfM introduced HSC as part of a broader PPH prevention and management package. The SfM project, in collaboration with the MOH, aimed to obtain valuable information from HCPs to determine whether the intervention led to improved knowledge and skills in the prevention of PPH and the use of HSC. The objectives of the study were to assess (1) HCP’s knowledge, perception, and experience of HSC in the selected health facilities and (2) the perspectives of policymakers on the introduction of HSC into the public health sector in Kenya.
Specific Aims
The specific aims were to document lessons learned from the introduction of HSC to the public sector in Kenya with the aim of using this experience to scale up the national introduction of HSC.
METHODS
Study Design
This was an interventional study design adopting a pre-post design for quantitative data and cross-sectional collection of qualitative data. Quantitative surveys were conducted at baseline (July 2021) and post-intervention (November 2022) to explore HCPs’ knowledge, perception, and experience of HSC. Qualitative data was collected at baseline only to understand the perspectives of policymakers on the introduction of HSC into the public health sector in Kenya.
Study Setting
The study recruited HCPs working in maternity and postnatal units in a mix of 39 urban/rural Comprehensive, Emergency Obstetric, and Neonatal Care (CEmONC) public health facilities from 10 out of the 47 counties at baseline and post-intervention. Selection was done by the MOH in consultation with SfM Project based on a situational analysis done by the United Nations Fund for Population Activities (UNFPA) on the burden of maternal deaths; their distribution in Kenya in the year 2014 found out that 15 out of 47 counties accounted for 98.7% of the total deaths in Kenya.[12] From this data, seven out of ten SfM-supported counties were selected: Nairobi, Nakuru, Kakamega, Kilifi, Migori, Kisumu, and Garissa. Additionally, Kitui County was included since it is identified as a high-priority county for improvement of maternal health indicators by the MOH as per the Kenya Demographic Health Survey data 2014. Finally, Kiambu and Tharaka Nithi counties were included in the list for regional balance.
In addition, policymakers involved in the introduction of lifesaving commodities, including uterotonics, at the national, county, sub county, and health facility levels were interviewed to gather their perspectives on the introduction of HSC in the public health sector.
Eligibility Criteria
At baseline, HCPs working in the maternity and offering services on the day of data collection were selected to respond to the questionnaire. At the end-line, HCPs working in the maternity units who had been exposed to project intervention packages and were present in their respective facilities were selected to respond to the quantitative questionnaire. Policymakers from national, county, sub county, and health facility levels were purposively selected to respond to the qualitative interviews. Policymakers are stakeholders with a position of responsibility related to maternal health, regulatory, and/or procurement of PPH medicines and/or programming policymakers comprised of the National Health Commodities manager, Kenya Medical Supplies Authority (KEMSA) maternal and neonatal health (MNH) focal person, county pharmacists, county reproductive health (RH) coordinators, county obstetrics/gynecologists, finance officers, procurement officers, health promotion officers, sub county RH coordinators, facility managers, and maternity in-charges.
Data Sources/Measurements
The HCP quantitative questionnaire was developed to assess HCP’s knowledge, experience, and perception before and after the study interventions. A key informant interview guide targeting policymakers was developed to understand health system factors that need to be considered for a successful introduction of HSC into the public health sector. The quantitative questionnaire was pretested among 20 HCPs, from public health facilities in two non-study counties, who met the inclusion criteria to establish respondent understanding of the question flow and willingness to respond. Trained research assistants used a mobile-based REDCap software (v5.26.4)[13] and audio recorders to collect the quantitative and qualitative data, respectively. Data quality checks were done in real-time on both quantitative and qualitative data. All data collected was stored in a password-protected SharePoint folder. To minimize potential bias, HCPs who met the eligibility criteria were randomly selected for quantitative interviews. Policymakers were purposively selected to include only those who had been involved in the introduction of HSC to provide insights based on their experience.
Study Variables
The study measured three outcome variables: (1) HSC knowledge, (2) HSC experience, and (3) HSC perception. The specific questions and statements clustered to measure each of the three outcome variables are presented in Table 1. Sociodemographics were considered as the independent variables to assess associations with the outcome variables.
| Questions/statements used | Considered responses | Outcome variables computations |
|---|---|---|
| HSC knowledge measurement questions | ||
| What is the recommended route of administration of HSC? | Intramuscular (IM) | HCPs who responded correctly to all 4 questions were categorized as having a ‘good’ knowledge of HSC. |
| What is the recommended dose of HSC? | 100 (micrograms) | |
| How many times would you administer HSC for prevention of PPH? | Single dose use | |
| At what temperature should HSC be stored and transported? | Room temperature (Below 30 degrees) | |
| HSC Experience measurement statements | 3-point Likert scale | |
| It is easy to distinguish HSC from other uterotonics from their packaging | Agree | HCPs who agreed to all 4 statements were categorized as having a ‘positive’ experience on HSC. |
| HSC has sustained effective contractions | Agree | |
| HSC can be stored at room temperature | Agree | |
| HSC has minimal side effects | Agree | |
| HSC perception measurement statements | 3-point Likert scale | |
| HSC is easy to administer | Agree | HCPs who agreed to all 2 statements were categorized as having a ‘positive’ perception on HSC. |
| HSC is effective in preventing PPH | Agree |
HSC: Heat-stable carbetocin, PPH: Postpartum hemorrhage, IM: Intramuscular, HCP: Healthcare provider.
Study Size
The sample size (n) for the number of HCPs interviewed was calculated using the Yamane sample size calculation formulae,[14] assuming a 5% desired level of accuracy (0.05) and the estimated number of HCPs (N = 300) to be trained in PPH prevention and management. Using the above formula, we yielded a sample size of 171 HCPs for the baseline survey with a post hoc power of 80%. The total sample (n = 171) was proportionately distributed across the 39 health facilities.
Statistical Analysis
Prior to data analysis, quantitative data cleaning was conducted to ensure completeness and consistency. Final analysis of quantitative data utilized STATA version 17.[15] Inferential statistics on quantitative data were done using chi-square tests to assess any significant changes in proportion between the baseline and end-line. Bivariate analyses assessed the characteristics of the sample at baseline and end-line. Multivariate logistic regression assessed differences between baseline and end-line for the outcome variables as well as associations of sociodemographic factors on the outcome variables. For qualitative data, audio recordings and notes generated in the interviews were used for transcription. The transcribed texts were analyzed using NVIVO 12.[16] The thematic framework was systematically applied to all the interview transcripts. To determine if saturation had been achieved, regularly reviewing relevant literature during the analysis process was done to ensure it adequately covered the themes emerging from the data. The diversity and representativeness of our sample were also assessed to ensure a broad range of participants or data sources with no new themes emerging. To ensure quality, the codes were clearly defined to guide the team involved and for ease of reference. Any inclusions or exclusions were discussed by the team to ensure refinement.
Ethical Approval
The study was approved by Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA, and Maseno University Scientific Ethical Review Committee (MUSEC), Kisumu city and state and country, and a research license obtained from the National Commission for Science, Technology, and Innovation, Nairobi, Kenya.
RESULTS
Sociodemographic Characteristics
A total of 171 HCPs selected across the 39 health facilities completed the survey both at baseline and end line. Nineteen policymakers from maternal health departments and supply chain agencies responded to the key informant interviews. The quantitative data collected at baseline and end line and the qualitative data collected at baseline were analyzed for this study.
At baseline, 74.9% were female and 91.2% were nurses and/or midwives. The key characteristics of the respondents at baseline and end line are summarized in Table 2. There was no significant difference between respondents’ characteristics at baseline and end line (p < 0.001).
| Baseline (n=171) | End-line (n=171) | P-values | |
|---|---|---|---|
| Frequency (%) | Frequency (%) | ||
| Age | |||
| Mean (SD) | 35.9 (9.19) | 35.5 (8.11) | |
| Median [Min, Max] | 34.0 [22.0, 59.0] | 34.0 [23.0, 65.0] | |
| Gender | |||
| Female | 128 (74.9%) | 120 (70.2%) | 0.333 |
| Male | 43(25.1%) | 51 (29.8%) | |
| Cadre* | |||
| Medical officers/ OB/GYN | 10 (5.9%) | 7 (4.1%) | 0.455 |
| Nurses/Midwives/ Registered Clinical officers | 161 (94.1%) | 164 (95.9%) | |
| Experience working in Maternity (years) | |||
| Mean (SD) | 5.5 (5.19) | 6.2 (5.07) | |
| Median [Min, Max] | 4.00 [0, 35.0] | 5.00 [0, 33.0] | |
| Work Experience Category | 0.684 | ||
| 0-5 Years | 105 (61.4%) | 94 (55.0%) | |
| 6-10 Years | 47 (27.5%) | 54 (31.6%) | |
| 11-15 Years | 12 (7.0%) | 15 (8.8%) | |
| >15 Years | 7 (4.1%) | 8 (4.7%) |
Main Variable Results
Quantitative findings
HCP Awareness of uterotonics
Respondents were asked to mention all uterotonics they were aware of. Uterotonics mentioned at baseline were oxytocin (99.4%), misoprostol (78.4%), and ergometrine (56.7%). There was a significant increase in the proportion of HCPs who were aware of HSC between baseline (18.1%, n = 171) and end line (100%, n = 171). There was no significant change in the proportion of HCPs who were aware of the other uterotonics between baseline and end line (p > 0.05).
HCP knowledge of HSC
Knowledge of HSC among the HCPs was assessed based on their understanding of the recommended route of administration, recommended dosage, frequency of administration for prevention of PPH, and the recommended storage or transportation temperatures. All attributes to measure knowledge of HSC significantly increased from baseline to end line (p < 0.001). At end line, 90.6% of HCPs correctly reported the recommended route of administration, correct recommended dose (98.9%), frequency of administration (98.8%), and correct temperature for storage and transportation (98.2%).
An 87% absolute increase in the proportion of HCPs reporting good knowledge, from 2–89% (odds ratio [OR] 448; 95 % CI: 130.0–1543.9), was identified from baseline to end line [Table 3]. Sociodemographic characteristics associated with “good” knowledge of HSC are listed in Table 4. HCPs with 11–15 years and over 15 years of professional experience had greater odds of good HSC knowledge than those with 0–5 years (AOR 3.34; 95 % CI: 0.9–12.4; and AOR 4.87; 95 % CI: 1.1–21.56, respectively). No other factors were significantly associated with “good” knowledge of HSC. Similarly, HCPs interviewed at the end-line had higher odds of “good” knowledge of HSC compared to baseline (AOR 708.94; 95% CI: 164.5–3055.4). No other factors were significantly associated with this outcome.
| Baseline (n=171) | End-line (n=171) | OR (95 % CI) | p-value | |
|---|---|---|---|---|
| % (n) | % (n) | |||
| HSC knowledge among HCPs | 1.75 (3) | 88.89 (152) | 448 (130.00, 1543.87) | <0.0001 |
| HSC experience among HCPs | 8.77 (15) | 89.47 (153) | 88.4 (43.00, 181.71) | <0.0001 |
| HSC perception among HCPs | 13.45 (23) | 91.23 (156) | 66.92 (33.62, 133.19) | <0.0001 |
HCP: Healthcare provider, HSC: Heat-stable carbetocin, OR: Odds’ ratio, CI: Confidence interval.
| HCPs knowledge on HSC | Experience in HSC among HCPs | HCPs perception on HSC | |
|---|---|---|---|
| AOR (95 % CI | AOR (95 % CI | AOR (95 % CI | |
| Age Category | |||
| 22-34 Years | Ref | Ref | Ref |
| 35-44 Years | 1.55(0.41,5.9) | 1.69(0.63,4.53) | 1.65(0.62,4.43) |
| 45-54 Years | 0.33(0.08,1.33) | 1.58(0.37,6.88) | 2.25(0.53,9.58) |
| 55+ Years | 0.26(0.03,2.3) | 1.52(0.25,9.34) | 1.78(0.34,9.35) |
| Gender | |||
| Female | Ref | Ref | Ref |
| Male | 0.88(0.31,2.51) | 1.62(0.61,4.28) | 1.32(0.54,3.28) |
| Professional Experience | |||
| 0-5 Years | Ref | Ref | Ref |
| 6-10 Years | 0.45(0.14,1.45) | 1.04(0.38,2.82) | 1.1(0.39,3.11) |
| 11-15 Years | 3.34(0.9,12.4)b | 2.05(0.64,6.62) | 1.23(0.41,3.72) |
| 15+ Years | 4.87(1.1,21.56)a | 2.34(0.22,24.86) | 2.5(0.48,13.1) |
| Cadre | |||
| Medical Officer/OB-GYN | Ref | Ref | Ref |
| Nurse/Registered clinical officers | 1.64(0.33,8.14) | 0.67(0.24,1.87) | 0.63(0.22,1.84) |
| Data Collection Time | |||
| Baseline | Ref | Ref | Ref |
| End-line | 708.94(164.5,3055.4)a | 100.44(46.42,217.34)a | 75.03(36.5,154.22)a |
HCP acceptability of HSC
To assess the acceptability of HSC among the HCPs, the respondents were asked to mention all the uterotonics they had administered to prevent PPH. At baseline, only 0.6% (n = 171) mentioned having administered HSC for the prevention of PPH compared to an end-line value of 98.8% (n = 171). Other uterotonic administered for the prevention of PPH at baseline included oxytocin (97.1%), misoprostol (70.2%), and ergometrine (28.1%). Other than HSC, the proportion of HCPs who reported having administered other uterotonics remained similar between baseline and end-line (p > 0.05).
HCP perception and experience of HSC
At the end-line, a higher proportion of HCPs agreed that HSC was easy to administer (93.6%), easy to distinguish from other uterotonics (91.8%), has sustained effective contractions (87.1%), can be stored at room temperature (93.6%), has minimal side effects (81.3%), and is effective in preventing PPH (91.2%). From baseline to end-line, reported experience and perception of HSC among the HCPs increased significantly by 80% (OR 88.4; 95 % CI: 43.0–181.7) and 78% (OR 66.9; 95% CI: 33.6–133.2), respectively, as shown in Table 3. HCPs interviewed at the end-line had higher odds of “positive” experience and “positive” perception of HSC compared to baseline (AOR 100.4; 95% CI: 46.4–217.3; and AOR 75.0; 95% CI: 36.5–154.2, respectively). No other sociodemographic factors were statistically significant with perception or experience of HSC.
Availability of PPH protocols/guidelines on the use of HSC
At baseline, 3.3% (n = 61) of HCPs were reported to have protocols/guidelines in place on the use of HSC at their health facilities compared to 96.5% (n = 171) at end line (p < 0.001), as shown in Figure 1.
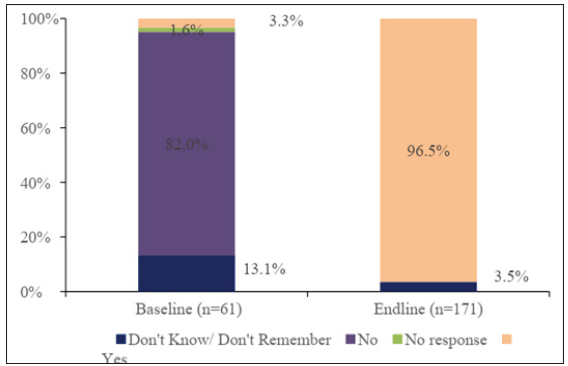
- Baseline versus end-line availability of protocols/guidelines in place on use of HSC. HSC: Heat-stable carbetocin, HCPs: Healthcare providers.
Qualitative Findings
Policymakers’ awareness of the 2018 WHO recommendations on uterotonic use for PPH prevention
In a cross-sectional assessment without the intervention, there was a higher level of awareness about the WHO recommendations on PPH prevention among policymakers than HCPs, with oxytocin being the most commonly mentioned uterotonic for PPH prevention. Information on HSC had been mostly obtained from project meetings at the county level. When asked about WHO/national guidelines dissemination in the public health system, the respondents felt that communication needs to be structured to improve the dissemination of information across different levels.
I think that preparation is very important, once the healthcare workers are taken through a very systematic introduction, .... they will be able to accept.
Policymaker, Nairobi County in urban Kenya
National and subnational involvement in the introduction of HSC in the public health sector
Policymakers from supply chain agencies shared the importance of the availability of structures at the county level to allow autonomy in budgeting for the purchase of supplies/commodities. They highlighted that leaders can be involved in advocating for funds to purchase uterotonics, including HSC.
There has to be a stakeholder to buy in because that’s the most important, especially at the National level…Counties must be fully convinced that this product will add value….
Policymaker, Kenya Medical Supply Chain Agency, Nairobi
The value proposition for the introduction of HSC into the public sector
The policymakers mentioned that there was value in the introduction of HSC into the public sector, especially in the lower-level facilities with cold chain challenges. Benefits cited included HSC not requiring cold chain storage as a benefit compared to oxytocin.
...facilities that do not have reliable cold storage facilities could really benefit from having HSC because of its effectiveness is assured...
Policymaker, Migori County, Kenya
Strengthening the supply chain to improve access to uterotonics, including HSC
Policymakers mentioned the need for proper inventory management as well as a sustainable financing mechanism for maternal health commodities to strengthen the supply chain.
...we need to have the commodity in place, ... have a stable supply chain, ... have sustainability frameworks for it. So that when dissemination is done there are no gaps...
Policymaker, National Level
Challenges in the introduction of HSC into the public health sector
Policymakers noted challenges related to budgetary allocation for maternal health commodities and suggested that they should be increased. The lack of adequate skills amongst HCPs who are the primary users was also highlighted as a challenge in the HSC introduction.
If the people who are supposed to use do not have the skills to use it […]. cost which hinders affordability […] the issue of supply chain efficiency, supplier unable to meet the demand and finally it comes down to monitoring issues in terms of safety…
Policymaker, National Level
DISCUSSION
This study aimed to understand PPH prevention knowledge, experience, and perspectives, including uterotonic use, by policymakers and HCPs in the public health sector within 10 counties in Kenya with a background of poor maintenance of existing cold chain for the main uterotonic in use, oxytocin. HSC, a quality assured heat stable drug, was introduced to mitigate these challenges.
Analyzed data from this study revealed that the sociodemographics of the respondents were not significantly associated with experience or perception of HSC at both baseline and end line. However, HCPs with 11–15 years and over 15 years of professional experience had greater odds of good HSC knowledge than those with 0–5 years. In general, HCPs with greater experience are most likely to receive more opportunities such as Basic Emergency Obstetric and Neonatal Care (BEmONC) training and orientation that reinforce their knowledge in PPH and uterotonics in general compared with those having fewer years of experience. They are also most likely to be stationed in one department compared to those with less experience who move around multiple departments. In comparison, a study conducted in Tanzania showed that the cadre of the respondents was significantly associated with knowledge levels in reference to nurses and clinical officers’ cadres.[17] At baseline, the analyzed data revealed that there were significant knowledge gaps in comparison to end-line in the domains of awareness and use of HSC, recommended route of administration, recommended dosage, frequency of administration for prevention of PPH, and the recommended storage/transportation temperatures across all the medical cadres. The results on recommended storage of HSC (p < 0.001) compared well to a study conducted in Nigeria that revealed poor knowledge of the storage of oxytocin.[8] Similarly, results from a qualitative systematic review of 35 studies in 29 countries compare well with our study, which reveals HCP knowledge gaps in PPH prevention and management, including the use of uterotonics.[18] The significant change in knowledge observed at end-line can be attributed to the intervention package. Our results are consistent with a rapid scooping review on implementing HSC for PPH prevention in low-resource settings, which showed that the introduction of HSC required ongoing support of HCP through training, mentorships, and supportive supervision to improve knowledge and confidence.[19] The facility-based training approach was associated with improved knowledge and skills saturation among HCPs. This approach allowed more HCPs to be trained within their workstation, ensuring minimal disruption of services, and inclusion of mentorship and follow-up structure.
Low knowledge of guidelines on the use of HSC at baseline can be explained by the fact that the national guidelines lacked HSC as a uterotonic for PPH prevention.[20] The increase in knowledge of the guidelines at end-line could be attributed to the dissemination of national obstetric and perinatal care guidelines and PPH protocols for HCPs.
The key informant interviews from policymakers generated valuable information on the successful introduction of HSC in the public health system. There is a need to collaborate with policymakers to integrate HSC into the national guidelines and clinical practice to tackle challenges related to the quality of existing uterotonics.[19] This calls for an overall health system assessment of barriers and enablers to secure the introduction and availability of HSC in the public sector.
The study results show that HCPs had favorable experiences with HSC use following the implementation of the intervention package. At the end line, more HCPs agreed that HSC was easy to administer (93.6%), easy to distinguish from other uterotonics (91.8%), can sustain effective contractions (87.1%), and its heat stability was highlighted during key informant interviews as a benefit in facilities without a reliable cold chain. These findings resonate well with a comparison study of HSC versus oxytocin in PPH prevention that the reported use of prophylactic carbetocin was associated with fewer PPH events.[21] Moreover, HSC was found to have minimal side effects (81.3%), and this concurs with the CHAMPION trial that revealed HSC has a safety profile that is comparable to oxytocin.[4]
The introduction of HSC should occur in the context of an integrated PPH prevention and management care approach, led and owned by HCPs. Engaging donors and stakeholders for support allows the country to achieve a wider scale-up of HSC.
Strengths and Limitations of the Study
The major strength of this study is the large and representative sample of respondents from different cadres of HCPs across the 10 counties which provided valuable insights into the feasibility and acceptability of HSC introduction. The study also carried out a qualitative component from policymakers to complement the quantitative findings. The major limitations include the study being affected by HCP transfers from maternity departments during the study period. Also, our study did not measure the outcome of PPH-associated deaths. Findings in this study were limited to comprehensive emergency obstetric and newborn care facilities since HSC availability in Kenya was limited only to hospitals as per national guideline recommendations. The study was also implemented in ten out of the 47 counties in Kenya.
CONCLUSION AND GLOBAL HEALTH IMPLICATIONS
The findings demonstrate that HCPs’ knowledge, perception, and experience coupled with the policymaker’s perspective are the key to the introduction of HSC in the public sector. Policymakers find value in introducing HSC as it alleviates challenges with the fragile cold chain systems. This study contributes to the global body of knowledge on the introduction of lifesaving commodities, which is anticipated to potentially improve PPH prevention and management, and hence reduce maternal mortality.
Key Messages
-
It is acceptable and feasible to introduce heat-stable carbetocin (HSC) in the public health sector. Healthcare providers (HCPs) had favorable experiences with HSC use during the implantation of the study. Reported experience and perception of HSC among the HCPs increased significantly from baseline to end-line.
-
Midwives and other HCPs can easily and safely provide HSC. Most HCPs agreed that HSC was easy to administer, easy to distinguish from other uterotonics, and could sustain effective contractions.
-
Policymakers find value in the introduction of HSC into the public sector. Its heat stability was highlighted during key informant interviews as a benefit in facilities without reliable cold chains.
Acknowledgments
None.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest
All authors of this study have no conflict of interest to declare.
Financial Disclosure
All authors of this study have no financial disclosure to make.
Funding/Support
The research in this publication was supported by funding from MSD, through its MSD for Mothers initiative, and is the sole responsibility of the authors. MSD for Mothers is an initiative of Merck & Co., Inc., Rahway, NJ, U.S.A.
Ethics Approval
This study was approved by the Maseno University Scientific Ethical Review Committee (MUSERC), approval #MUERC/00965/21 and Johns Hopkins Bloomberg School of Public Health, approval #16971.
Declaration of Patient Consent
Patient’s consent not required as there are no patients in this study.
Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
None.
Special Collection
This article is published as part of the special collection on prevention and treatment of postpartum hemorrhage in high-burden low- and middle-income countries: building cross-national evidence through implementation research.
REFERENCES
- Maternal mortality Evidence brief. :1-4.
- Saving Mothers’ Lives: Confidential Enquiry into Maternal Death First Report Policy Brief. 2017;242:0-3.
- [PubMed]
- WHO Recommendations: Uterotonics for the prevention of postpartum haemorrhage. World Health Organization; 2018. p. :53. [Accessed 2024 Jan 11]. Available from: http://apps.who.int/bookorders.%0Ahttps://www.who.int/reproductivehealth/publications/uterotonics-pph/en/
- Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med.. 2018;379(8):743-52.
- [CrossRef] [PubMed] [Google Scholar]
- Uterotonic drugs for the prevention of postpartum haemorrhage: A cost-effectiveness analysis. Pharmacoecon Open.. 2019;3(2):163-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Translating clinical practice guidelines into practice. AARC Times.. 2016;40(3):5-7. [Accessed 2024 Jan 11]. Available from: http://edgehill.idm.oclc.org/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=113572264&login.asp&site=ehost-live&scope=site
- [Google Scholar]
- The effect of Kenya’s free maternal health care policy on the utilization of health facility delivery services and maternal and neonatal mortality in public health facilities. BMC Pregnancy Childbirth.. 2018;18(1):1-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A descriptive study of healthcare-providers’ experiences with the use and quality of oxytocin for the prevention of post-partum hemorrhage in Nigeria: A nation-wide survey. PLoS One.. 2021;16(10):1-17.
- [Google Scholar]
- A synthesis of clinical and health system bottlenecks to implementing new WHO postpartum hemorrhage recommendations: Secondary data analysis of the kenya confidential enquiry into maternal deaths 2014–2017. Int J Gynaecol Obstet.. 2022;158(S1):14-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality of oxytocin available in low- and middle-income countries: A systematic review of the literature. BJOG.. 2016;123(13):2076-86.
- [CrossRef] [PubMed] [Google Scholar]
- Global causes of maternal death: A WHO systematic analysis. Lancet Glob Health.. 2014;2(6):323-33.
- [Google Scholar]
- Summary Report of the Assessment of UNFPA’s Advocacy Campaign to End Preventable Maternal and New-Born Mortality in Kenya List of Abbreviations. 2019. p. :1-20.
- Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform.. 2009;42(2):377-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Statistics, An introductory analysis 2nd ed.
- College Station T 77845 U. Stata Corp (2021) Stata Statistical Software: Release 17. 2021. [Accessed 2024 Jan 11]. Available from: https://www.stata.com
- Health care providers’ knowledge of clinical protocols for postpartum hemorrhage care in Kenya: A cross-sectional study. BMC Pregnancy Childbirth.. 2022;22(1):1-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- What matters to women and healthcare providers in relation to interventions for the prevention of postpartum haemorrhage: A qualitative systematic review. PLoS One.. 2019;14(5):1-23.
- [CrossRef] [Google Scholar]
- Implementing heat-stable carbetocin for postpartum haemorrhage prevention in low-resource settings: A rapid scoping review. Int J Environ Res Public Health.. 2022;19(7):3765.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Midwives’ perceptions towards the ministry of health guidelines for the provision of immediate postpartum care in rural health facilities in Uganda. . 2023;7:1-16.
- [Google Scholar]
- Cost-effectiveness analysis of carbetocin versus oxytocin for the prevention of postpartum hemorrhage following vaginal birth in the United Kingdom. J Med Econ.. 2022;25(1):129-37.
- [CrossRef] [PubMed] [Google Scholar]





