Translate this page into:
Integrating Heat-Stable Carbetocin and Tranexamic Acid for Prevention and Management of Postpartum Hemorrhage in Sub-Saharan Africa: A Five-Country Pilot Implementation Study

*Corresponding author: Sara Rushwan, MPH, PhD, Concept Foundation, Avenue de Sécheron, Geneva, Switzerland. Tel: +41 22 734 2560 s.rushwan@conceptfoundation.org
-
Received: ,
Accepted: ,
How to cite this article: Rushwan S, Forna F, Abubeker FA, Tufa T, Millogo T, Nakalembe M, Adu-Bonsaffoh K, et al. Integrating heat-stable carbetocin and tranexamic acid for prevention and management of postpartum hemorrhage in Sub-Saharan Africa: A five-country pilot implementation study. Int J MCH AIDS. 2024;13:S15-27. doi: 10.25259/IJMA_34_2024
Abstract
Background and Objective
Globally, postpartum hemorrhage (PPH) remains the most common direct cause of maternal mortality. This study evaluated the feasibility and acceptability of introducing heat-stable carbetocin (HSC) for PPH prevention and tranexamic acid (TXA) for PPH treatment in five Sub-Saharan African countries following recent World Health Organization (WHO) recommendations. This study also assessed healthcare providers’ (HCPs’) favorability toward using these medicines.
Methods
We conducted a mixed methods pilot implementation study in selected facilities across Burkina Faso, Ethiopia, Ghana, Sierra Leone, and Uganda between May and December 2022. We compared baseline data obtained from patient registers with data collected during implementation on the safe and appropriate use of HSC and TXA using descriptive statistics. HCP responses were analyzed qualitatively using a thematic analysis.
Results
Following training, HSC was administered prophylactically in 11,329 (92.4%) of 12,262 deliveries in all study facilities which received a uteorotonic for PPH prevention during implementation and was used safely and appropriately. TXA administration for PPH treatment was done safely, appropriately, and within the WHO-recommended time. No adverse events were reported throughout the study. HCPs overall showed high confidence in, and favorability toward, using both medicines.
Conclusion and Global Health Implications
Our study demonstrated that HSC and TXA can be safely and appropriately implemented in primary and tertiary facilities, and their introduction is feasible and acceptable from the perspective of HCPs. A holistic approach to training and regular supportive supervision is needed to ensure the continued safe use of these new and lesser-utilized PPH medicines. Dedicated training is required to improve the documentation of patient charts on PPH care. Introducing these medicines holds promise for improving PPH care in low- and middle-income countries, including by addressing suboptimal efficacy due to cold chain system challenges.
Keywords
Postpartum Hemorrhage
Heat-Stable Carbetocin
Tranexamic Acid
Sub-Saharan Africa
Implementation Research
INTRODUCTION
Background of the Study
Postpartum hemorrhage (PPH), defined as blood loss of 500 ml or more after birth, is the leading direct cause of maternal mortality globally, responsible for about a quarter of all maternal deaths, and disproportionately impacts women in Sub-Saharan Africa (SSA) and South Asia (∼80%).[1,2] The most important component to prevent PPH is the use of uterotonics like oxytocin and misoprostol during active management of the third stage of labor (AMTSL). Oxytocin has been used for decades for the prevention and treatment of PPH, and it remains the drug of choice for both indications according to the World Health Organization (WHO) recommendations.[3]
However, substantive progress to reduce maternal mortality is lagging behind meeting the Sustainable Development Goal 3.1 target by 2030. In SSA countries, the burden of PPH is compounded by the use of substandard or falsified medicines. A systematic review assessing studies on the quality of oxytocin in low- and middle-income countries (LMICs) found a high prevalence of substandard oxytocin and misoprostol, and many LMICs face issues with maintaining a consistent cold chain and transport conditions for oxytocin use in maternity care, which impacts its efficacy.[4]
Thus, in the absence of oxytocin or when its quality cannot be assured, heat-stable carbetocin (HSC) has been recommended for PPH prevention in the 2018 WHO recommendations.[5] HSC is a long-acting uterotonic that has been shown to be non-inferior to oxytocin and may be more effective for some outcomes in the prevention of PPH without an increase in side effects.[6,7] HSC is stable for a minimum of three years at 30°C without refrigeration and therefore holds promise for use in countries with cold chain challenges.[8] Currently, HSC is recommended for PPH prevention only, and to date, there is limited published evidence of HSC use in resource-challenged settings. A recent rapid scoping review on implementing HSC in low-resource settings showed limited evidence of healthcare provider (HCP) acceptability in lower-level maternity care, such as in Basic Emergency Obstetric and Newborn Care (BEmONC) facilities in rural areas as well as the health system’s capacity for an HSC-inclusive PPH intervention package.[9]
Meanwhile, tranexamic acid (TXA) is an antifibrinolytic agent that has been available since the early 1960s and is indicated in several hemorrhage-related conditions for controlling bleeding.[10] In 2017, following the publication of the WOMAN trial results, WHO recommended intravenous TXA administration for PPH treatment as an adjunct therapy to oxytocin and other standard treatment options.[11,12] Subsequently, both HSC and TXA were included in the 2019 WHO Essential Medicines List (EML).[13]
Despite the 2017 and 2018 WHO recommendations, both drugs are still underused or not used at all for PPH prevention and management in many countries with a high burden. HSC is currently being registered in LMICs with overall limited availability. TXA is available in many countries but is not registered for the PPH treatment indication. HSC and TXA are not yet widely used in many LMICs and there is variability among countries as to whether these drugs are recommended or included in the national EML.
The transition from recommendations to practice is contingent upon multiple steps along the product introduction pathway, including the update of national policies and consistent availability of quality-assured, affordable medicines. Between 2019 and 2022, Concept Foundation implemented a project to accelerate national availability and end-user access to HSC and TXA in 15 SSA countries by supporting Ministries of Health in updating their national guidelines, EMLs, and essential packages of health services to include these medicines, as well as developing clinical protocols on PPH prevention and management with supportive job aids. Dissemination of global recommendations is insufficient on its own to change country practice, hence, implementation research is important in helping to bridge the gap between evidence and practice in real-world settings to support the integration and scale-up of new interventions into national health systems. Efforts to reduce PPH-related morbidity and mortality require these real-world evidence-based recommendations to transition into national adoption of clinical policies and practice.
The five countries included in this study were part of the 2019–2022 Concept Foundation project and all have relatively high maternal mortality ratios (per 100,000 live births): Burkina Faso (198), Ethiopia (267), Ghana (263), Sierra Leone (443), and Uganda (284).[14,15] The countries’ Ministries of Health, in an attempt to implement the 2017 and 2018 WHO PPH recommendations, updated their latest national guidelines to include HSC and TXA. The latest EMLs in Burkina Faso, Ghana, Sierra Leone, and Uganda include these two medicines (Ethiopia’s EML currently includes TXA and will be updated to include HSC). Despite these advances, neither medicine is widely available in any of the countries. To generate evidence on introducing new and lesser-used WHO-recommended medicines in high PPH burden settings, this implementation research study assessed the feasibility, acceptability, and appropriate use of HSC for PPH prevention and TXA for PPH treatment in five SSA countries.
Objectives of the Study
Our objective was to assess the safe and appropriate use of HSC and TXA for PPH prevention and management in high-burden settings.
Specific Aims
Our specific aims were to assess the feasibility and acceptability of introducing HSC and TXA into routine care at BEmONC and Comprehensive Emergency Obstetric and Newborn Care (CEmONC) facilities in five SSA countries.
METHODS
Study Setting and Design
We conducted a pre-post study in 19 facilities from five countries (Burkina Faso, Ethiopia, Ghana, Sierra Leone, and Uganda). During the study period (May 1 to December 31 2022), we collected baseline data retrospectively from patient registers in the labor ward for two months at each facility. Data collected included uterotonics use patterns, patient outcomes, HCP knowledge, attitudes, and practices (KAP) pertaining to PPH prevention and management, and facility characteristics related to the availability of electricity and obstetric medicines stock-out. All identifiable patient data was anonymized. We selected a convenience sample of two BEmONC and two CEmONC facilities from each country, one each from an urban and a rural area. Uganda was the only country with one BEmONC facility due to its large number of deliveries, hence a total of nine BEmONC and ten CEmONC facilities were included in the study. The facilities were selected based on willingness and ability to integrate HSC for PPH prevention and TXA for PPH treatment into routine care. Carbetocin Ferring (100 µg/ml, Ferring Pharmaceuticals) and Tranexamic Acid (100 mg/ml, Medochemie Ltd) were supplied by IDA Foundation, Netherlands, to each of the study countries.
In all study countries, HCPs in the selected facilities were trained on the safe and appropriate use of HSC and TXA using a mix of theoretical and practical simulation training. Following the training, both medicines were introduced into each facility, which marked the beginning of data collection for the two-month implementation period (Ghana was the exception, whereby data collection was extended for two weeks due to a slower-than-normal delivery rate). There was regular supportive supervision throughout the implementation phase. All patient data were extracted from data that were routinely collected in clinical practice. The questionnaires did not contain any identifiable patient information.
For the KAP assessment, we purposively recruited a sample of HCPs from each health facility. Maximum variation sampling was used to achieve a stratified sample without random selection and to ensure heterogeneity of research participants. In each of the selected facilities, HCPs were sampled based on their cadres, such as doctor/midwife or nurse/nurse aide.
Statistical Analysis
The clinical data were entered into REDCap software and analyzed using descriptive statistics in the form of mean (± standard deviation) for continuous variables, and proportion (for categorical variables) expressed as a percentage, as described in the literature.[16] The KAP questionnaire had multiple-choice questions which were also analyzed descriptively. The open-ended questions were analyzed qualitatively through thematic analysis to identify emerging themes from the responses using Dedoose software version 9.0. This method is comprehensively used for analyzing qualitative data.[17]
Ethical Approval and Informed Consent
Institutional Review Board approval was obtained in all countries before the start of the study. We employed broad participation criteria to be as inclusive as possible of all cadres of HCPs. All potential participants received information about the study in the national language, conforming to ethical requirements for research involving human subjects. Those who consented to participate in the study were requested to sign the informed consent form and it was made clear that they were free to withdraw from the study at any stage without risk of any negative consequences. The clinical data were retrieved from patient records and no contact was made with women delivering during the study period.
RESULTS
Summary of Total Number of Deliveries Captured by Facility Type
During the study period, 25,116 deliveries were recorded across the five countries, out of which 12,153 were captured during the baseline period and 12,963 during the implementation period. There was a total of 2,309 deliveries at BEmONCs and 22,807 deliveries at CEmONCs.
Facility Characteristics
Overall, 55.6% of the BEmONCs reported electricity being available sometimes and 44.4% reported electricity being available all the time. Half of the BEmONCs reported refrigeration being sometimes available for oxytocin and the other half reported availability all the time, and 88.9% reported no stock-out of oxytocin over a six-month period.
As for the CEmONC facilities, 15.0% reported electricity being available sometimes and 85.0% reported electricity being available all the time. A quarter of the CEmONCs reported refrigeration being sometimes available for oxytocin and 75.0% reported availability all the time, and 90.0% reported no stock-out of oxytocin over a six-month period.
Obstetric Characteristics
Out of the 25,116 recorded deliveries during the study period, the median age of women who delivered was 27 years. Throughout the study, the percentage of nulliparous deliveries in all study BEmONCs ranged from 14.5% (n = 11) in Ethiopia to 37.4% (n = 92) in Ghana, while nulliparous deliveries at CEmONCs ranged from 28.2% (n = 574) in Ghana to 55.1% (n = 2287) in Uganda. There was 100% vaginal delivery in BEmONCs of all countries at baseline and during implementation. In all CEmONCs, the proportion of cesarean deliveries ranged from 23.3% (n = 293) in Burkina Faso to 37.7% (n = 678) in Ethiopia at baseline, and from 13.6% (n = 237) in Burkina Faso to 43.2% (n = 2104) in Uganda during implementation. During baseline and implementation, uterotonic administration for labor induction/augmentation at BEmONCs was <4% in Ethiopia, Ghana, Sierra Leone, and Uganda. In CEmONCs, uterotonic administration for labor induction/augmentation ranged from 4.7% to 43.6% at baseline and from 2.9% to 27.1% during implementation in Ethiopia, Ghana, Sierra Leone, and Uganda. Burkina Faso results are not included due to unavailable data. A summary of the main demographic characteristics captured is presented in Table 1.
| Burkina Faso | Ethiopia | Ghana | Sierra Leone | Uganda | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEmONC | CEmONC | BEmONC | CEmONC | BEmONC | CEmONC | BEmONC | CEmONC | BEmONC | CEmONC | ||||||||||||
| B | I | B | I | B | I | B | I | B | I | B | I | B | I | B | I | B | I | B | I | ||
| Total number. of deliveries captured in the study | 253 | 367 | 1262 | 1735 | 92 | 76 | 1803 | 1869 | 229 | 246 | 2052 | 1805 | 130 | 129 | 1621 | 1481 | 399 | 388 | 4312 | 4867 | |
| Maternal age (years) | n | 248 | 358 | 1222 | 1602 | 92 | 76 | 1787 | 1861 | 228 | 246 | 2037 | 1797 | 128 | 127 | 1545 | 1476 | 392 | 386 | 4283 | 4832 |
| Mean | 26.6 | 26.3 | 27.2 | 27.2 | 27.3 | 26.7 | 27.2 | 27.6 | 28.1 | 28.3 | 29.8 | 29.6 | 24.1 | 26.1 | 25.6 | 25.6 | 26.1 | 25.3 | 26.2 | 26.4 | |
| STD | 6.2 | 5.8 | 6.2 | 6.3 | 4.9 | 4.8 | 5.0 | 4.9 | 5.3 | 5.6 | 5.9 | 5.7 | 4.6 | 5.5 | 5.6 | 5.7 | 4.6 | 4.9 | 5.6 | 5.8 | |
| Nulliparous | n | 57 | 89 | 369 | 558 | 19 | 11 | 733 | 726 | 66 | 92 | 574 | 587 | 35 | 25 | 567 | 529 | 75 | 88 | 2287 | 2530 |
| N | 230 | 354 | 1158 | 1637 | 92 | 76 | 1782 | 1861 | 227 | 246 | 2033 | 1785 | 130 | 129 | 1550 | 1477 | 390 | 385 | 4152 | 4756 | |
| % | 24.8 | 25.1 | 31.9 | 34.1 | 20.7 | 14.5 | 41.1 | 39.0 | 29.1 | 37.4 | 28.2 | 32.9 | 26.9 | 19.4 | 36.6 | 35.8 | 19.2 | 22.8 | 55.1 | 53.2 | |
| Uterotonic administered for labor induction/augmentation | n | 0 | 0 | 420 | 424 | 1 | 5 | 97 | 52 | 1 | 0 | 704 | 402 | 14 | 10 | 613 | 479 | ||||
| N | 92 | 75 | 1790 | 1861 | 229 | 246 | 2049 | 1803 | 130 | 129 | 1616 | 1481 | 399 | 388 | 4302 | 4867 | |||||
| % | 0.0 | 0.0 | 23.5 | 22.8 | 0.4 | 2.0 | 4.7 | 2.9 | 0.8 | 0.0 | 43.6 | 27.1 | 3.5 | 2.6 | 14.2 | 9.8 | |||||
| Total mode of delivery | N | 252 | 365 | 1259 | 1735 | 91 | 75 | 1797 | 1852 | 229 | 246 | 2051 | 1805 | 130 | 129 | 1618 | 1481 | 399 | 388 | 4300 | 4867 |
| Vaginal birth | n | 252 | 365 | 964 | 1496 | 91 | 75 | 1119 | 1117 | 229 | 246 | 1364 | 1147 | 130 | 129 | 1053 | 935 | 399 | 388 | 2677 | 2756 |
| % | 100 | 100 | 76.6 | 86.2 | 100 | 100 | 62.3 | 60.3 | 100 | 100 | 66.5 | 63.5 | 100 | 100 | 65.0 | 63.1 | 100 | 100 | 62.2 | 56.6 | |
| Cesarean section | n | 0 | 0 | 293 | 237 | 0 | 0 | 678 | 735 | 0 | 0 | 686 | 658 | 0 | 0 | 564 | 545 | 0 | 0 | 1600 | 2104 |
| % | 0.0 | 0.0 | 23.3 | 13.6 | 0.0 | 0.0 | 37.7 | 39.7 | 0.0 | 0.0 | 33.4 | 36.5 | 0.0 | 0.0 | 34.9 | 36.8 | 0.0 | 0.0 | 37.2 | 43.2 | |
Data are presented as n (%) or mean (STD). B = Baseline and I = Implementation
A summary of the obstetric characteristics of the study countries, including maternal age, proportion of nulliparous women, uterotonic use for labor induction/augmentation, and mode of delivery. The n (%) represents the number of cases and the proportion as a percentage, with the N denominator being the total number of deliveries captured within the study period.
Main Outcome Results
Quantitative Analysis
Appropriate use of HSC
Uterotonic administration for PPH prevention by country, facility type, and study phase
The percentage of women receiving prophylactic uterotonics at baseline was in general very high in all countries and in both facility types (most were more than 90%). Therefore, there was no room for improvement after implementation, except in Uganda CEmONCs, which improved from 79.9% to 90.9%. In Burkina Faso, there was a 14% reduction in prophylactic uterotonic administration in CEmONC facilities. During implementation, HSC was the most used uterotonic for PPH prevention in both BEmONCs and CEmONCs. In BEmONCs, HSC was administered to >99% of women in all study countries. In CEmONCs, HSC was administered to >97% of women in Sierra Leone, Burkina Faso, and Ghana, and 87% in Ethiopia and Uganda. These findings are presented in Figures 1 and 2. No adverse events were reported from the use of HSC during implementation.
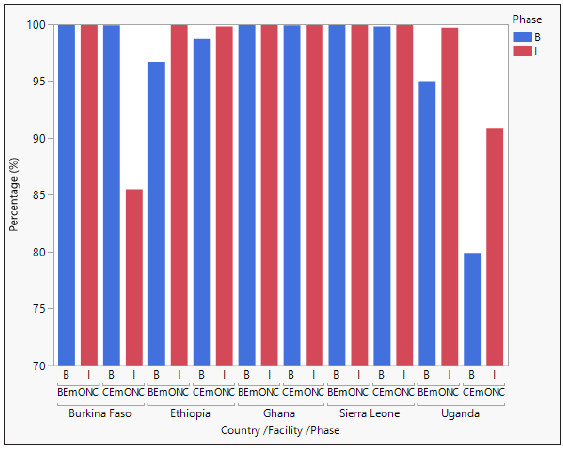
- Uterotonic administration for PPH prevention by country, facility type, and study phase.
- Data are presented as percentage of prophylactic uterotonic administered at BEmONC and CEmONC facilities in each study country at baseline (pre-intervention) and during implementation (post-intervention). At baseline, the uterotonics administered were either oxytocin, misoprostol, or oxytocin in combination with misoprostol. During implementation, the uterotonics administered were either HSC, oxytocin, misoprostol, oxytocin in combination with misoprostol, HSC in combination with oxytocin or misoprostol, or carboprost. B = Baseline and I = Implementation
- *HSC was administered in combination with other uterotonics for PPH prevention to 3.1% (55) of women in CEmONCs in Ghana, 0.5% (7) in Burkina Faso, 0.5% (22) in Uganda, and 0.1% (1) in Sierra Leone. Across all BEmONCs, one patient in Ghana received HSC in combination with other uterotonics. In Ghana, 54 cases in CEmONCs and 1 case in BEmONCs were administered HSC in combination with misoprostol for PPH prevention.
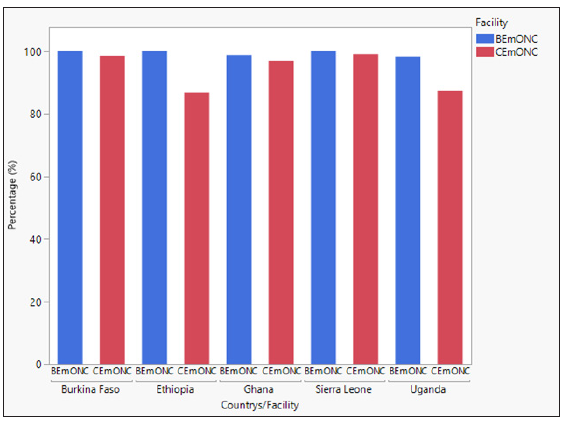
- HSC administration for PPH prevention during study implementation by country and facility type.
- Data are presented as percentage of HSC administered at BEmONC and CEmONC facilities in each study country during implementation.
Additional uterotonic administration for PPH following prophylaxis
Additional uterotonics are administered for continued or heavy bleeding after prophylactic uterotonics have been given. Overall, additional uterotonic use was low in all study countries. In BEmONCs, additional uterotonics were used in ≤5% of women in Ethiopia, Sierra Leone, and Uganda at baseline. During implementation, the use of additional uterotonics decreased in Uganda. No additional uterotonic use was recorded in Ethiopia and Sierra Leone during implementation, and also at baseline and implementation phases in Ghana. In Burkina Faso, additional uterotonic data was not captured in patient records.
In CEmONCs, additional uterotonics were used in ≤10% of women in Ethiopia, Uganda, Sierra Leone, and Ghana at baseline. There was a general decrease in the use of additional uterotonics in these countries during implementation, except for Ghana which remained the same. Data for additional uterotonic use were not available in Burkina Faso. Oxytocin, misoprostol or oxytocin in combination with misoprostol were the most common additional uterotonics used in all countries. HSC was not administered as an additional uterotonic during implementation in all study facilities. The findings are summarized in Figure 3.
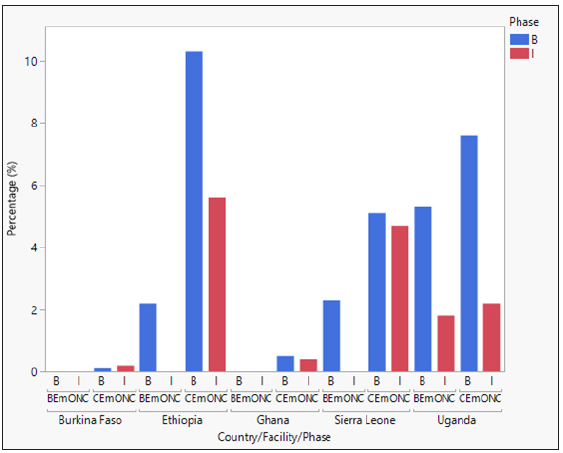
- Additional uterotonic administration by country, facility type, and study phase.
- Percentage of additional uterotonic administered at the study facilities at baseline (pre-intervention) and implementation (post-intervention). B = Baseline and I = Implementation
Appropriate use of TXA
TXA administration for PPH treatment by country, facility type, and study phase
At baseline, TXA was only readily accessible in Ghana, Ethiopia, and Uganda. Burkina Faso and Sierra Leone did not have TXA readily accessible.
Once TXA was made available during implementation, use in BEmONCs and CEmONCs without prior availability was at ≤2% in Burkina Faso, and ≤8% in Sierra Leone. In Uganda and Ethiopia CEmONCs, there was minimal to no difference in TXA administration. In Ghana, TXA use decreased in both facility types during implementation. There were no cases of TXA administration in Ethiopia BEmONCs at baseline or during implementation, and only three cases (0.8%) of TXA administration in Uganda BEmONCs during implementation. The results are demonstrated in Table 2. No adverse events were reported from the use of TXA during implementation.
| Burkina Faso | Ethiopia | Ghana | Sierra Leone | Uganda | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEmONC | CEmONC | BEmONC | CEmONC | BEmONC | CEmONC | BEmONC | CEmONC | BEmONC | CEmONC | ||||||||||||
| B | I | B | I | B | I | B | I | B | I | B | I | B | I | B | I | B | I | B | I | ||
|
TXA administered for PPH treatment Time from delivery to TXA administration for PPH treatment (mins) |
n | 0 | 8 | 0 | 18 | 0 | 0 | 2 | 23 | 10 | 4 | 88 | 22 | 0 | 8 | 0 | 120 | 0 | 3 | 219 | 243 |
| N | 252 | 366 | 1256 | 1732 | 92 | 76 | 1802 | 1863 | 229 | 246 | 2049 | 1799 | 130 | 129 | 1618 | 1480 | 399 | 388 | 4302 | 4867 | |
| % | 0.0 | 2.2 | 0.0 | 1.0 | 0.0 | 0.0 | 0.1 | 1.2 | 4.4 | 1.6 | 4.3 | 1.2 | 0.0 | 6.2 | 0.0 | 8.1 | 0.0 | 0.8 | 5.1 | 5.0 | |
|
Time from delivery to TXA administration for PPH treatment (mins) |
n | 0 | 8 | 0 | 18 | 0 | 0 | 2 | 23 | 10 | 4 | 88 | 22 | 0 | 8 | 0 | 109 | 0 | 3 | 39 | 192 |
| Median | - | 28.0 | - | 31.5 | - | - | 121.0 | 26.0 | 10.5 | 20.5 | 11.0 | 9.5 | - | 57.5 | - | 24.0 | - | 12.0 | 55.0 | 7.0 | |
| IQR | - | 30.3 | - | 24.8 | - | - | 182.0 | 29.0 | 8.0 | 38.8 | 10.8 | 8.8 | - | 51.8 | - | 27.5 | - | 5.0 | 229.0 | 19.8 | |
Proportion of TXA administration for PPH treatment. The n (%) represents the number of women who received TXA and the proportion as a percentage, with the N denominator being the total number of deliveries captured within the study period. Time from delivery to TXA administration in minutes. The n represents the number of cases with recorded TXA administration. Data are presented as n (%) or median (IQR). B = Baseline and I = Implementation
Time from delivery to TXA administration for PPH treatment in BEmONC and CEmONC facilities
Once TXA was made available during implementation, the median time from delivery to TXA administration for PPH treatment in Faso and Sierra Leone BEmONCs and CEmONCs (and Uganda BEmONCs)was less than one hour. In Ethiopia and Uganda CEmONCs, there was a clear decrease from baseline to implementation in the time from delivery to TXA administration (and a slight decrease for Ghana CEmONCs). In Ghana BEmONCs, there was a ten-minute increase in the median during implementation. Overall, in the sites with TXA available at baseline, the median time of administration was within 30 minutes during implementation. The results are demonstrated in Table 2.
Blood transfusion and other PPH treatments received
In CEmONCs, 58 women in Burkina Faso (4.8%) received blood transfusions at baseline and 75 (4.3%) during the implementation period (the reduced percentage during implementation is due to the 40% increase in the number of delivery records captured). Seventeen women (1.0%) were transfused at baseline and 12 (0.6%) during implementation in Ethiopia; 24 (1.5%) were transfused at baseline and 23 (1.5%) during implementation in Sierra Leone; and 76 (1.8%) were transfused at baseline and 39 (0.8%) during implementation in Uganda. Ghana results are not included due to unavailable data. There were no blood transfusions recorded in BEmONCs of all study countries at baseline or during implementation.
In CEmONCs, the intrauterine tamponade was used only at baseline and in three countries—two patients in Burkina Faso, three in Ethiopia, and three in Uganda. A non-pneumatic anti-shock garment (NASG) was used in one patient at baseline in Uganda and one patient during the implementation phase in Ethiopia. No intrauterine tamponade or NASG was used in any BEmONC facility during the study period.
Safe use of HSC and TXA
Monitoring of heat-stable carbetocin and tranexamic acid ampoules
Facilities monitored ampoule counts for potential misuse of HSC and TXA and recorded the number of ampoules that were supplied to them, the number administered to patients during implementation, and the number remaining at the end of study implementation. They also recorded the number of ampoules of HSC and TXA that were broken or wasted, missing, or used for reasons other than PPH prevention or treatment.
For HSC, no countries reported use for reasons other than PPH prevention. For TXA, Ethiopia, Sierra Leone, and Uganda reported the use of the medication for reasons other than PPH treatment—nine patients in Ethiopia (0.7%) received TXA empirically to prevent PPH and eight patients in Sierra Leone (2.0%)received TXA empirically to prevent PPH (five), or for gynecologic surgery (two), and for a patient undergoing surgery for a motor vehicle accident (one). Uganda reported three cases of TXA administered to prevent PPH in high-risk patients.
Healthcare provider KAP assessment
Healthcare provider methods of measuring blood loss
We asked a sample of 140 HCPs to report on how they measure blood loss, and the cumulative result from the five countries showed that 75.0% of HCPs visually estimate blood loss, which remained unchanged post-implementation. The results are presented in Table 3.
| Methods of measuring blood loss | Burkina Faso | Ethiopia | Ghana | Sierra Leone | Uganda | Baseline Total | Implementation Total | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | n | n (%) | n (%) | n (%) | ||||||
| B | I | B | I | B | I | B | I | B | I | B + I | |||
| I don’t measure blood loss | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 (0.7%) | 0 | 1 (0.4%) |
| I visually estimate blood loss | 20 | 19 | 23 | 21 | 35 | 35 | 17 | 20 | 10 | 9 | 105 (75.0%) | 104 (74.3%) | 209 (74.6%) |
| I measure the volume of blood collected in drapes in milliliters | 2 | 4 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 5 (3.6%) | 6 (4.3%) | 11 (3.9%) |
| I measure by weighing sponges/packs soaked with blood | 1 | 1 | 3 | 5 | 1 | 0 | 3 | 2 | 1 | 2 | 9 (6.4%) | 10 (7.1%) | 19 (6.8%) |
| I measure the volume of blood in drapes + weighing sponges/packs soaked with blood | 1 | 1 | 3 | 2 | 2 | 2 | 2 | 1 | 2 | 3 | 10 (7.1%) | 9 (6.4%) | 19 (6.8%) |
| I measure by counting the number of sponges/packs used | 2 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 1 | 2 | 10 (7.1%) | 11 (7.9%) | 21 (7.5%) |
| I visually estimate + count the number of sponges/packs used | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| I do something else | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TOTAL | 26 | 27 | 32 | 31 | 41 | 41 | 26 | 25 | 15 | 16 | 140 | 140 | 280 |
Answers from self-reported methods of measuring blood loss by a sample of 140 healthcare providers collected during baseline and implementation. Data are presented as n (%).
Healthcare provider hesitations with using HSC and/or TXA
We asked the same sample of HCPs in each country a yes/no question on whether they had any hesitation with using HSC and/or TXA. Overall, HCPs had no hesitation about using HSC or TXA post-implementation in all countries, except Burkina Faso where there is a slight increase in the proportion of self-reported hesitation with using TXA post-implementation. The results are shown in Table 4. The reasons for self-reported hesitations at baseline and during implementation are expanded upon in the thematic analysis.
|
Burkina Faso n (%) |
Ethiopia n (%) |
Ghana n (%) |
Sierra Leone n (%) |
Uganda n (%) |
Baseline Total n (%) |
Implementation Total n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat-stable carbetocin | B | I | B | I | B | I | B | I | B | I | |||
| Hesitations using heat-stable carbetocin | 5 (16.7%) | 0 | 17 (60.7%) | 0 | 0 | 0 | 10 (38.5%) | 0 | 8 (50.0%) | 0 | 40 (28.6%) | 0 | |
| No hesitations using heat-stable carbetocin | 25 (83.3%) | 30 (100%) | 11 (39.3%) | 28 (100%) | 40 (100%) | 40 (100%) | 16 (61.5%) | 26 (100%) | 8 (50.0%) | 16 (100%) | 100 (71.4%) | 140 (100%) | |
| TOTAL | 30 | 30 | 28 | 28 | 40 | 40 | 26 | 26 | 16 | 16 | 140 | 140 | |
| Tranexamic acid | |||||||||||||
| Hesitations using tranexamic acid | 4 (13.3%) | 5 (16.7%) | 10 (35.7%) | 1 (3.6%) | 0 | 0 | 8 (30.8%) | 0 | 1 (6.3%) | 0 | 23 (16.4%) | 6 (4.3%) | |
| No hesitations using tranexamic acid | 26 (86.7%) | 25 (83.3%) | 18 (64.3%) | 27 (96.4%) | 40 (100%) | 40 (100%) | 18 (69.2%) | 26 (100%) | 15 (93.7%) | 16 (100%) | 117 (83.6%) | 134 (95.7%) | |
| TOTAL | 30 | 30 | 28 | 28 | 40 | 40 | 26 | 26 | 16 | 16 | 140 | 140 | |
Add Yes/No answers from self-reported hesitations with using heat-stable carbetocin for PPH prevention and tranexamic acid for PPH treatment by a sample of 140 healthcare providers collected during baseline and implementation. Data are presented as n (%).
Qualitative analysis
Using an inductive thematic analysis, three distinct but related themes were identified from responses by a sample of 140 HCPs (the findings are captured in Table 5).
| Theme | Example quotes/issues identified | Occurrence in countries | Study phase |
| Need for capacity building and training on PPH prevention and management | Capacity building on PPH prevention and management | All countries | Baseline + Implementation |
| Need for continuous professional development on PPH prevention and management | All countries | Baseline + Implementation | |
| Training on AMTSL | Ethiopia, Ghana | Baseline | |
| Lack of training on using uterine balloon tamponade | Ethiopia, Ghana, Sierra Leone, Uganda | Baseline + Implementation | |
| Lack of training on new drugs like HSC | All countries | Baseline | |
|
“I would like to train on how to detect and assess PPH very early” Training on early detection of PPH risk factors |
Ethiopia, Ghana | Baseline + Implementation | |
| Multilevel challenges in preventing and treating PPH | Uterotonic/PPH medicine unavailability or shortage | All countries | Baseline + Implementation |
| Drug shortage of HSC and TXA | All countries | Implementation | |
| Lack of knowledge about new medicines | All countries | Baseline | |
| Knowledge gap amongst staff in preventing and managing PPH | All countries | Baseline + Implementation | |
|
Difficulty in identifying PPH patients Problem with early identification of risk factors of PPH |
All countries | Baseline + Implementation | |
|
Lack of adequate staff Manpower shortage |
Ethiopia, Ghana, Uganda | Baseline + Implementation | |
|
Poor cold chain system Inadequate cold chain for oxytocin storage |
Ethiopia, Uganda | Baseline + Implementation | |
|
Underestimation of maternal blood loss Misdiagnosis—no standard means of measuring blood loss |
Ethiopia, Ghana, Sierra Leone, Uganda | Baseline + Implementation | |
| Poor documentation—history, interventions done, time records | Uganda | Baseline | |
| Lack of training and availability of HSC and TXA |
Lack of knowledge about the products “We don’t have the drugs and don’t know how to use them” |
All countries | Baseline |
| Concern about the effectiveness of HSC | Ethiopia | Baseline | |
| Lack of training and availability of the drugs | All countries | Baseline + Implementation | |
| Deepening knowledge of HSC | Burkina Faso | Implementation | |
| “Further training on use of TXA because the administration time is long, the product requires great caution” | Burkina Faso | Implementation |
Results from thematic analysis of HCP responses to challenges faced in PPH care and hesitations around using HSC and/or TXA.
Need for capacity building and training on PPH prevention and management
HCPs from all countries highlighted the need for capacity building and refresher training (to tackle knowledge gaps) on PPH prevention and management. They also listed areas of training that they would like to receive which included appropriate AMTSL, how to estimate the amount of blood loss, and how to identify mothers at high risk of PPH.
Multilevel challenges in preventing and treating PPH
HCPs in all countries highlighted a multitude of challenges in preventing and/or treating PPH that span from medicine/staff shortages to identifying PPH. For example:
HCPs in all countries also shared potential solutions to prevent and treat PPH that mainly focused on increased training, capacity, and availability of medicines. Proposed solutions included the “availability of uterotonic agents which are not dependent on cold chain” and “early identification of those at risk of PPH”.“Overwhelming number of obstetric emergencies compared to the staff number”
“Drug stock-outs, not having enough blood products, inadequate cold chain for oxytocin storage, low empowerment among midwives in the management of PPH”
Lack of training and availability of HSC and TXA
The hesitations identified by HCPs at baseline pertained to a lack of knowledge and availability of HSC and TXA. The only hesitations identified post-implementation were specific to Burkina Faso and related to improving knowledge of HSC and training on TXA use “because the administration time is long, the product requires great caution”.
DISCUSSION
Our pilot implementation study in five SSA countries demonstrated the feasibility and acceptability of integrating HSC and TXA into routine care following HCP training. The two medicines were safely and appropriately used according to WHO recommendations in all study countries in the majority of women. We included BEmONC and CEmONC facilities to assess the feasibility of introduction in different contexts. There were no additional interventions except for the focused training before implementation.
Although the objective of the study was to evaluate the integration of the two medicines into routine care, the decision to administer both drugs was made by the HCPs and not reinforced for the study. The data capture for routine care was generally weak, especially in some BEmONC facilities where the only recording is the large registers and each patient’s data is captured in one row. We tracked all ampoules of HSC and TXA deployed at the study sites. The results were reassuring, with no leakage or contraindicated use of HSC for labor induction or augmentation. The low rates of uterotonic administration for labor induction and augmentation in BEmONCs (including no cases in Burkina Faso) is in line with national guidance, which recommends against administering uterotonics for labor induction and augmentation in facilities without surgical capability. The low rates of uterotonic use for labor induction and augmentation in Ghana CEmONCs are also in line with standard practice.
A somewhat unexpected finding was the extent of oxytocin use in combination with misoprostol for PPH prevention in Ghana at baseline. During implementation, we found that the highest number of cases (55) of HSC used in combination with other uterotonics for PPH prevention was in Ghana CEmONCs where 54 of these cases were used in combination with misoprostol; however, there were no adverse events reported. Although we emphasized the use of HSC alone in the training, the result could be related to their standard practice of combined administration of oxytocin and misoprostol for PPH prevention, which was recorded in 99% of cases in BEmONCs and CEmONCs at baseline (Figure 1). Across all BEmONCs, there was only one case of a patient receiving HSC in combination with other uterotonics in Ghana, which is an interesting finding on how well the training was adhered to between the two facility types. This is supported by the finding that switching to HSC for PPH prevention was higher in BEmONC facilities of all countries. In Ethiopia and Uganda, 13.0% and 8.1% of patients, respectively, continued to receive oxytocin during the implementation phase at CEmONC facilities (Figure 2). It is also worth noting that additional uterotonic administration was generally low and HSC was appropriately not administered as an additional uterotonic.
Prior to implementation, most study facilities had no or limited access to TXA because it was either not available, considered expensive, or lacked awareness of TXA use for PPH treatment. TXA administration during implementation was aligned with the WHO recommendation of administering the first dose of TXA within 30 minutes.[12] TXA use was generally low in all facilities during implementation. Since TXA was available at baseline in Ghana and used in facilities for PPH treatment, reduced use during implementation could indicate that there were fewer diagnosed cases of PPH that required treatment with TXA. Incidents of blood transfusion generally decreased during implementation in the study countries (except Ghana where data for blood transfusions was unavailable), which could correlate with increased TXA use. The uptake of PPH devices such as intrauterine tamponade and NASG was nonexistent or very low, which implies issues related to availability, access, and/or training in the study countries.
No adverse events were reported from the use of either medicine in all countries. The need to accurately document the timing of administration was emphasized during the pre-implementation training as a measure of appropriate use. Previous studies have shown an underestimation of uterotonic provision due to the quality of documentation.[18–19] We recommend continued reinforcement of accurate documentation of uterotonic administration and assessment of adherence. The same is recommended for TXA administration as it has been reported that a reduced effect of TXA was observed the longer the delay in administration.[20]
The qualitative data findings identified the need for capacity building and training on the use of HSC and TXA within a holistic training model of PPH care. This was highlighted by HCPs in all countries, and based on our study findings we strongly recommend, as with all maternal health medicines, refresher training with supportive supervision. The training on safe and appropriate use and integration of the two medicines into routine care was not perceived as difficult by the trainers or the HCPs receiving the training. The lack of medicine availability was a recurring theme in all countries. This speaks to the ramifications of a lack of end-to-end planning for the introduction of maternal health medicines that impede their availability and access. Although our facility questionnaire data showed over 80% reporting of no stock-out of oxytocin over a six-month period in BEmONCs and CEmONCs across the five countries, it is a snapshot indication of stock status for one uterotonic and not reflective of the facility’s product stock-out history.
HCP acceptance of the medicines was observed quantitatively through the percentage of HSC administration for PPH prevention and TXA use for PPH treatment, and qualitatively through no self-reported hesitation with using either medicine post-implementation in Ethiopia, Ghana, Sierra Leone, and Uganda. Anecdotally, HCPs in all study countries praised the two medicines, with noteworthy descriptions such as “game-changers” and “life-savers.” There was a slight increase in HCP hesitations in Burkina Faso over the use of TXA, and the reasons included the slow administration of the drug which requires caution, especially in women with low blood pressure whereby rapid infusion may exacerbate hypotension. The slow administration was also perceived as delaying strict guidance in BEmONCs on the immediate referral of patients with bleeding to CEmONC facilities.
Another aspect we were interested in gauging from HCP practices was the most common method of measuring blood loss, as subjective assessment persists across countries with high PPH burden leading to underestimated blood loss.[21] The patient data collected showed an increase in PPH diagnoses during implementation in Burkina Faso, Sierra Leone, and Uganda. This could be associated with the pre-implementation training; however, the overall low rates of PPH clinical diagnosis (<10%) across all countries throughout the study period are indicative of issues with diagnosing and/or possible reluctance to record a PPH case. Also, we found that 75% of HCPs sampled across the five countries visually estimate blood loss. This self-reported finding and wide recognition in the literature around the pervasive issue of visual blood loss estimation necessitates dedicated training sessions to implement the timely and recently published WHO guideline on objective blood loss assessment for early detection of PPH.[22]
Strengths and Limitations of the Study
We assessed the feasibility of concurrently integrating two new and lesser-used PPH medicines into clinical care in primary and tertiary facilities of five SSA countries. We were able to identify similarities and differences between countries and facility types, which we believe will be helpful in health systems’ decision-making. We demonstrated that despite data recording limitations, HSC and TXA were safely and appropriately implemented in all sites.
Labor induction data for Burkina Faso was unavailable for CEmONCs due to poor record capture (as is the case for unavailable blood transfusion data in Ghana). Overall, poor recording of obstetric conditions and interventions in all study countries is a major limitation of our study. We only collected the data recorded in patient records and birth registers and did not triangulate or confirm use by asking the HCPs. The duration of data capture was short, which is a limitation, but the sample size is large enough to provide useful information on safe and appropriate use.
CONCLUSION AND GLOBAL HEALTH IMPLICATIONS
We demonstrated that HSC and TXA integration into immediate postpartum care can be done safely and appropriately in BEmONC and CEmONC facilities and that the use of both medicines was accepted by HCPs. The fragility of cold chain integrity and storage was a real challenge for all of the countries, highlighting the importance of more widespread use of heat-stable medicines and supplies to strengthen the quality of PPH care. We believe health information systems need to be dramatically improved for reliable data capture, especially in BEmONC settings where recordkeeping is practically difficult to capture all events during childbirth.
We also believe that introduction efforts, including national policy change, EML inclusion, registration, procurement of quality-assured products, clinical protocol development, and onsite training, are all crucial for successful implementation and scale-up. Monitoring of appropriate use and pharmacovigilance should accompany all implementation efforts. To reduce the global burden of PPH-related maternal mortality, the adoption of a health systems approach, inclusive of the elements listed above, is paramount.
Key Messages
HSC and TXA can be safely and appropriately integrated into immediate postpartum care in cold-chain challenged settings
Refresher training that encompasses a holistic approach to PPH care and regular supportive supervision is needed to ensure continued safe and appropriate use of HSC and TXA
Health information systems need to be improved for accurate record capture of all events during childbirth
Acknowledgments
We would like to thank all study facilities for participating in the research, particularly the local data collectors who dedicated a lot of time and effort to this study. We would also like to thank all the healthcare providers who took part in the questionnaire and gave their time to help us understand their perspectives. Also, a special thank you to Vania Nilsson and Luciana Abreu (Statistika) for analyzing the data.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest
The authors declare no competing interests.
Financial Disclosure
Nothing to declare.
Funding/Support
The research in this publication was supported by funding from MSD through its MSD for Mothers initiative and is the sole responsibility of the authors. MSD for Mothers is an initiative of Merck & Co., Inc., Rahway, NJ, USA.
Ethics Approval
The study received ethical approval from the Institutional Review Boards in Burkina Faso, Ethiopia, Ghana, Sierra Leone, and Uganda. The authorization to conduct the study and utilize its results in reports and publications was acquired from the responsible national authority in all the health facilities involved in the research.
Declaration of Patient Consent
We employed broad participation criteria to be as inclusive as possible of all cadres of HCPs. All potential participants received information about the study in the national language, conforming to ethical requirements for research involving human subjects. Those who consented to participate in the study were requested to sign the informed consent form, and it was made clear that they were free to withdraw from the study at any stage without risk of any negative consequences. The clinical data were retrieved from patient records and no contact was made with women delivering during the study period.
Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Disclaimer
Concept Foundation led the research in collaboration with the principal investigators in the study countries.
Special Collection
This article is published as part of the special collection on prevention and treatment of postpartum hemorrhage in high-burden low- and middle-income countries: building cross-national evidence through implementation research.
REFERENCES
- Global causes of maternal death: A WHO systematic analysis. Lancet Glob Health.. 2014;2(6):e323-33. https://doi.org/10.1016/S2214-109X(14)70227-X
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet.. 2016;388(10053):1775-812. https://doi.org/10.1016/s0140-6736(16)31470-2
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO recommendations on uterotonics for postpartum haemorrhage prevention: What works, and which one? BMJ Glob Health.. 2019;4(2):e001466.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality of oxytocin available in low- and middle-income countries: A systematic review of the literature. BJOG.. 2016;123(13):2076-86.
- [CrossRef] [PubMed] [Google Scholar]
- WHO recommendations on uterotonics for the prevention of postpartum haemorrhage. Geneva: WHO; 2018. [Accessed 2023 Nov 24]. https://www.who.int/publications/i/item/9789241550420
- Heat-Stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med.. 2018;379(8):743-52.
- [CrossRef] [PubMed] [Google Scholar]
- Uterotonic agents for preventing postpartum haemorrhage: A network meta-analysis. Cochrane Database Syst Rev.. 2018;4(4):CD011689.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development and stability of a heat-stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle-income countries. J Pept Sci.. 2018;24(6):e3082.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Implementing heat-stable carbetocin for postpartum haemorrhage prevention in low-resource settings: A rapid scoping review. Int J Environ Res Public Health.. 2022;19(7):3765.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr.. 2017;129(9-10):303-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet.. 2017;389(10084):2105-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage. Geneva: World Health Organization; 2017. [Accessed 2024 Apr 15]. https://www.who.int/publications/i/item/9789241550154
- WHO model list of essential medicines – 21st list, 2019. Geneva: WHO; 2019. [Accessed 2023 Nov 24]. https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06
- Trends in maternal mortality 2000 to 2020: estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. Geneva: WHO; 2023. [Accessed 2023 Nov 24]. https://www.who.int/publications/i/item/9789240068759
- The Demographic Health Survey Program, Institut National de la Statistique et de la Démographie. Burkina Faso Enquête Démographique et de Santé. 2023. Accessed 2023 Nov 24]. Available from: https://dhsprogram.com/publications/publication-FR378-DHS-Final-Reports.cfm
- Statistical methods and reasoning for the clinical sciences: Evidence-based practice. Plural Publishing; 2014.
- A step-by-step process of thematic analysis to develop a conceptual model in qualitative research. Int J Qualitative Methods.. 2023;22 Available from: https://doi.org/10.1177/16094069231205789
- [CrossRef] [Google Scholar]
- Uterotonic use immediately following birth: Using a novel methodology to estimate population coverage in four countries. BMC Health Serv Res.. 2015;15:9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Uterotonics for prevention of postpartum haemorrhage: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth.. 2021;21(Suppl 1):230.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet.. 2010;391(10116):125-132. Available from: http://dx.doi.org/10.1016/S0140-6736(17)32455-8
- [Google Scholar]
- Randomized trial of early detection and treatment of postpartum hemorrhage. N Engl J Med.. 2023;389(1):11-21.
- [CrossRef] [PubMed] [Google Scholar]
- World Health Organization. WHO recommendation on the assessment of postpartum blood loss and use of a treatment bundle for postpartum haemorrhage. Geneva: World Health Organization; 2023. [Accessed 2024 Apr 20]. https://www.who.int/publications/i/item/9789240085398





