Translate this page into:
A Novel Combined Mother-Infant Clinic to Optimize Post-Partum Maternal Retention, Service Utilization, and Linkage to Services in HIV Care in Rural Rwanda
*Corresponding author email: gneza1@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background and Objectives:
Despite recent improvements in accessibility of services to prevent mother-to-child transmission of HIV, maternal retention in HIV care remains a challenge in the post-partum period. This study assessed service utilization, program retention, and linkage to routine services, as well as clinical outcomes for mothers and infants, following implementation of an integrated mother-infant clinic in rural Rwanda.
Methods:
We conducted a retrospective cohort study of all HIV-positive mothers and their infants enrolled in the integrated clinics in two rural districts between July 1, 2012, and June 30, 2013. At 18 months post-partum, data on mother-infant service utilization and program outcomes were reported.
Results:
Of the 185 mother-infant pairs in the clinics, 98.4% of mothers were on antiretroviral therapy (ART) and 30.3% used modern contraception at enrollment. At 18 months post-partum, 98.4% of mothers were retained and linked back to adult HIV program. All mothers were on ART and 72.0% on modern contraception. For infants, 93.0% completed follow-up. Two (1.1%) infants tested HIV positive.
Conclusion and Global Health Implication:
An integrated clinic was successfully implemented in rural Rwanda with high mother retention in care and low mother to child HIV transmission rates. This model of integration of services may contribute to improved mother-infant retention in care during post-partum period and should be considered as one approach to addressing this challenge in similar settings.
Keywords
HIV
Integrated Clinic
Combined Clinic
Antiretroviral Therapy
Post-partum Retention
Linkages
ART
PMTCT
Option B+
Africa
1. Background and Objectives
Of the 1.2 million new HIV infections among women worldwide in 2014, 66% occurred in sub-Saharan Africa (SSA),[1] and an estimated 600 children were infected with HIV daily through mother-to-child transmission (MTCT).[1] While the expanded coverage of prevention of mother-to-child HIV transmission (PMTCT) services and the introduction of the “Option B+” for life-long antiretroviral therapy (ART) for pregnant mothers with HIV have greatly reduced vertical transmission in this region,[2] many challenges remain in the elimination of MTCT and effective ART service delivery for women and children.[3]
Challenges to effective PMTCT and maternal outcomes include the linkage of women into HIV care and treatment services at the time of HIV diagnosis and retention in care during key transition points throughout pre-partum and post-partum periods.[4] Integration of maternal and child health (MCH) services has been proposed as one method to improve retention of mothers and infants in care, particularly during the critical post-partum period,[5] which accounts for the greatest attrition from routine HIV care.[6] During the post-partum period, mothers face the dual challenge of accessing appropriate care for themselves and for their HIV-exposed infants, who are at higher risk for morbidity and mortality than their non-HIV exposed counterparts.[7] Post-partum services for maternal HIV care and PMTCT for exposed infants are rarely integrated,[8] leading to poor adherence and high attrition from care after delivery and during the breastfeeding period.[9] Experience with integrating mother and infants’ services, clinical outcomes from integrated services, particularly beyond six weeks post-partum, and linkage to routine care after PMTCT period are limited and rarely described in the literature.[10]
The Rwanda Ministry of Health with support from a non-governmental organization, Partners In Health (PIH), developed and implemented a novel model integrating mother-infant HIV care services called the “Combined Clinic”. The Combined Clinic aimed to decrease the care seeking burden on post-partum HIV-positive mothers through integration of maternal and infant care, thereby reducing the number of clinic visits, transport costs, and time away from other duties, such as employment and child care responsibilities. This study assessed utilization of the Combined Clinic services, program retention, linkage to routine services after the clinic and outcomes for mothers and infants enrolled in the clinic.
2. Methods
2.1. Study setting
Rwanda, a country of approximately 11.3 million people in Eastern Africa, had an overall HIV prevalence of 3.0% in 2015 with a higher prevalence (4.0%) among women of childbearing age.[11] In 2010, Rwanda national guidelines adopted lifelong ART for all pregnant women with HIV infection and established a goal of reducing MTCT to <2% by 2015.[12] Nationally reported MTCT rates at 18 months post-partum declined from 6.7% in 2011[13] to 1.8% in 2014,[14] primarily due to improved access to decentralized PMTCT services and improvements in early infant diagnosis via dried blood spot testing.[15] Both ART and PMTCT services are offered at 97% of primary health centers throughout the country, which also provide basic maternal and child health (MCH) services including antenatal care (ANC), immunizations, family planning (FP) and management of acute childhood illness.[14]
According to the Rwanda national protocol, HIV-positive pregnant women are identified through routine voluntary counselling and testing services during their first ANC visit or upon positive pregnancy testing within the HIV care and treatment program. These women are provided integrated ANC and PMTCT services throughout pregnancy, and following delivery are referred to continue routine HIV care and treatment at an adult ART clinic. HIV-exposed infants are also provided follow-up care for 18 months post-partum at a separate PMTCT clinic within the same health facility. The follow-up care includes administration of nevirapine prophylaxis until 6 weeks of age, cotrimoxazole from 6 weeks to 18 months of age, routine screening for malnutrition and infections, and HIV testing at 6 weeks, 9 months, and 18 months of age. ART and PMTCT services are located in the same health facility, but typically not integrated in terms of staffing or clinic room.
In the Combined Clinic model, mother and infant services are delivered at a single point of care until infants reach 18 months of age (Figure 1). Mothers enrolled in the Combined Clinic in facilities included in this study were also enrolled in the community-based accompaniment program (CBAP) supported by PIH. Under CBAP, patients receive daily home visits from lay treatment partners trained as community health workers who deliver and monitor treatment uptake. The CBAP intervention and related outcomes have been previously described in detail.[16] Additionally, mothers enrolled in Combined Clinics received nutritional supplementation via a monthly food package, consisting of 4 kg of porridge mixture (maize, soybean and sorghum) and 1 kg of sugar. Mothers with a documented viral load above >1000 copies/ml were provided with an option of artificial milk formula feeding, which included a monthly ration of 1.2 to 2.4 kg of artificial milk powder until the infant is 9 months old, in addition to the standard monthly distribution of the porridge mixture and sugar. Mothers provided with artificial milk formula also received stoves, fuel, and clean canisters for artificial milk preparation. Clinic nurses demonstrated proper breastfeeding and artificial milk preparation techniques. The Combined Clinic utilized an electronic medical record (EMR) system, which generated monthly alerts to clinicians for delayed patient visits and other clinically significant notices.
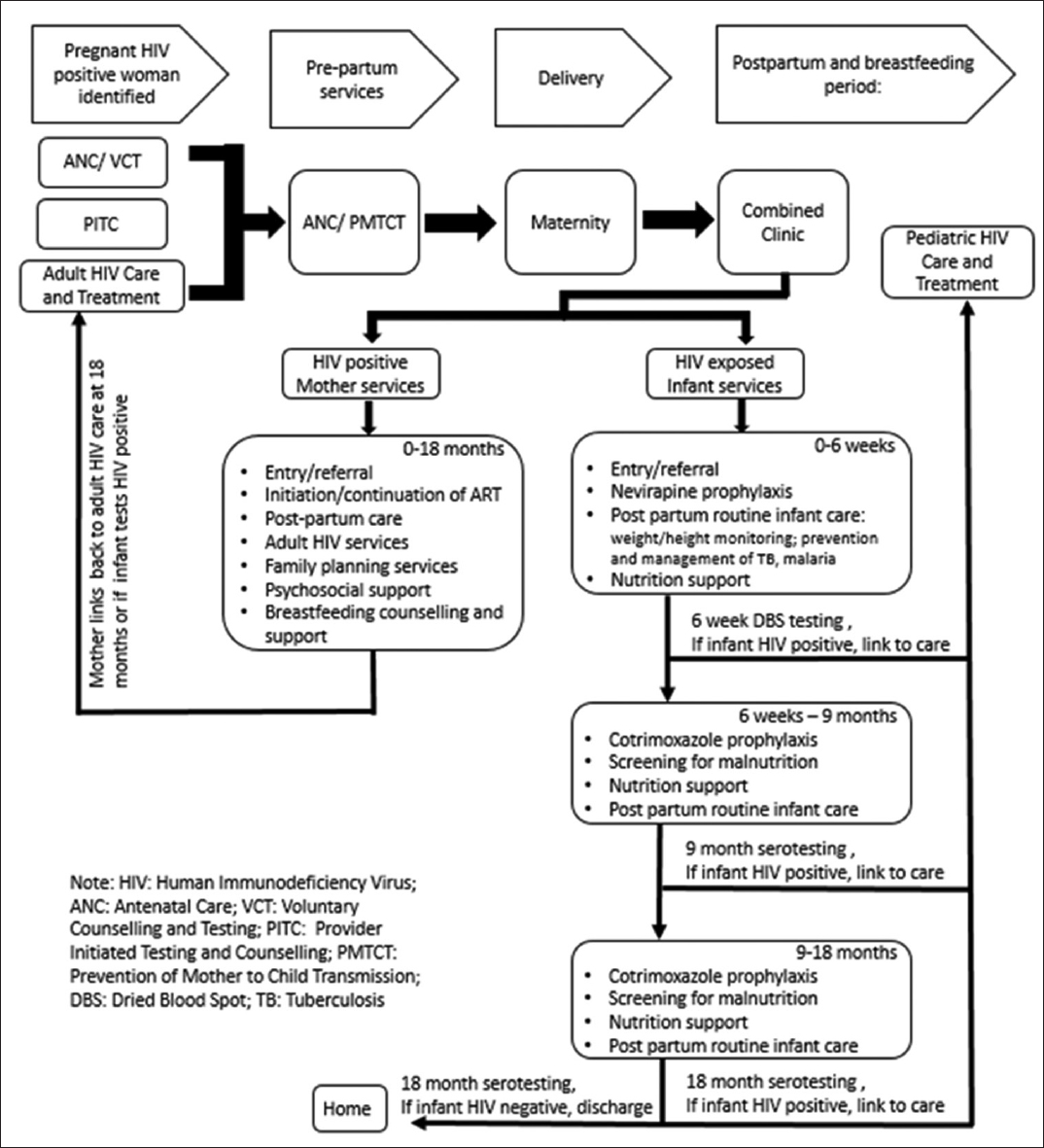
- The “Combined Clinic” Model and Services for Mother and Infants
2.2. Study population, data collection and analysis
We conducted a retrospective cohort study of HIV-positive mothers and their infants aged 0-18 months enrolled in Combined Clinics in all 23 health centers in Southern Kayonza and Kirehe Districts between July 1, 2012, and June 30, 2013. Eligible mothers and infants were identified using the EMR system. Mothers and infants enrolled in the clinic but whose records could not be matched to confirm mother-infant relationship were excluded from the study. We extracted data on mother-infant pairs including demographics, service utilization and outcomes from patient charts and registers. Maternal and infant loss-to-follow-up was defined as lack of a clinic visit after 15 months post-partum. Infant HIV test was considered done if both test date and test results were recorded in the charts, and an infant’s definitive HIV diagnosis was based on the date and results of the last HIV test recorded in the charts. Infant use of nevirapine and test at six weeks was limited to those enrolled before two months of age and test at 18 months of age excluded those who died, transferred or lost to follow up. Acute and chronic malnutrition was measured by z-scores for weight-for-height and height-for-age, respectively, and analysed according to the World Health Organization 2006 Child Growth Standards where z-score < -3 was considered severe acute or chronic malnutrition, -2 to -3 was moderate acute or chronic malnutrition, -1 to -2 was mild acute or chronic malnutrition, and >-1 was no acute or chronic malnutrition.[17] We performed descriptive analyses using Stata v13 (College Station, TX: StataCorp LP). The Rwanda National Health Research Committee (Kigali, Rwanda), Rwanda National Ethics Committee (Kigali, Rwanda) and Partners Institutional Review Board (Boston, USA) approved this study. We also received data collection permission from the heads of the facilities.
3. Results
3.1. Mother and infant care utilization patterns
From July 1, 2012, to June 30, 2013, 185 mother-infant pairs enrolled in the Combined Clinic in Southern Kayonza and Kirehe Districts (Table 1). At enrollment, 55.8% of mothers (n=101 of 181) aged between 25 and 34 years, 30.3% (n=57) were using a modern FP method (injectable contraceptives, oral contraception, condoms and implants), and 98.4% (n=182) were documented to be receiving ART. For the infants, 89.4% (n=135 of 151) were delivered through vaginal delivery, and 83.3% (n=140 of 168) were enrolled between birth and two months of age. In regards to service utilization, 97.1% (n=136 of 140) and 89.7% (n=166) infants were documented to have received nevirapine and cotrimoxazole, respectively, according to national guidelines (Table 2). In addition, 95.1% (n=176) infants had at least one documented HIV test result during their time in the program with 69.3% (n=97 of 140) having documented test result at six weeks (0-2 months), 63.8% (n=118) at 9 months (+/-3 months) and 87.8% (n=151 of 172) at 18 months (≥15 months).
| Variables | N=185 | |
|---|---|---|
| n | % | |
| Mother’s age | N=181 | |
| 17-24 years | 34 | 18.8 |
| 25-34 years | 101 | 55.8 |
| 35-45 years | 46 | 25.4 |
| Mother’s family planning status | ||
| Natural method | 21 | 11.4 |
| Modern method† | 57 | 30.3 |
| Method unspecified | 4 | 4.9 |
| No family planning | 104 | 56.2 |
| Mother on anti-retroviral therapy | ||
| Yes | 182 | 98.4 |
| Not reported | 3 | 1.6 |
| Infant age at enrollment | N=168 | |
| Enrolled at birth | 87 | 51.8 |
| 1-2 months | 53 | 31.5 |
| 3-12 months | 21 | 12.5 |
| >12 months | 7 | 4.2 |
| Infant gender | N=175 | |
| Male | 86 | 49.1 |
| Female | 89 | 50.9 |
| Infant delivery method | N=151 | |
| Spontaneous vaginal/breech delivery | 135 | 89.4 |
| Caesarean section | 16 | 10.6 |
| Infant birth location | N=71 | |
| Health facility | 67 | 94.4 |
| Home | 4 | 5.6 |
| Variables | N=185 | |
|---|---|---|
| n | % | |
| Infant on nevirapine‡ | N=140 | |
| Yes | 136 | 97.1 |
| No | 4 | 2.9 |
| Infant on cotrimoxazole | ||
| Yes | 166 | 89.7 |
| No | 10 | 5.4 |
| Not reported | 9 | 4.9 |
| Infant with HIV test result recorded†† | ||
| Yes | 176 | 95.1 |
| No | 9 | 4.9 |
| Infant received HIV test at 6 weeks (0-2 months)‡ | N=140 | |
| Yes | 97 | 69.3 |
| No | 38 | 27.1 |
| Not reported | 5 | 3.6 |
| Infant received HIV test at 9 months (+/-3 months) | ||
| Yes | 118 | 63.8 |
| No | 58 | 31.3 |
| Not reported | 9 | 4.9 |
| Infant received HIV test at 18 months (≥15 months)† | N=172 | |
| Yes | 151 | 87.8 |
| No | 12 | 7.0 |
| Not reported | 9 | 5.2 |
3.2. Linkage to services and outcomes
Following completion of the 18 months post-partum follow-up, 98.4% of mothers (n=182) were discharged from the Combined Clinic and linked back to an adult HIV program, one (0.5%) mother died, and two (1.1%) transferred outside of the catchment area (Table 3). No mother was lost-to-follow-up. Of the 182 mothers completing the Combined Clinic time, at discharge 72.0% (n=131) were using a modern FP method and 100% were on ART. For the infants, 93.0% (n=172 of 185) completed 18 months of follow up, eight (4.3%) transferred out of the catchment area, four (2.2%) were lost-to-follow-up and one (0.5%) died. Of the 176 infants with HIV test results, two (1.1%) tested HIV positive. Of the 166 (94.3%) infants whose last HIV test was negative, 92.2% (n=153) had their last test at ≥15 months and 6.0% (n=10) had their last test between 6-14 months. Eight (4.5%) infants had indeterminate diagnosis. For 95 infants with anthropometrics recorded at the time of discharge for acute malnutrition, one (1.1%) met criteria for severe acute malnutrition and three (3.2%) for moderate acute malnutrition. For 93 infants with anthropometrics recorded for chronic malnutrition, 28 (30.1%) had severe chronic malnutrition and 26 (28.0%) had moderate chronic malnutrition
| Variables | n | % |
|---|---|---|
| Mother outcome | ||
| Completed 18 months’ follow-up and linked back to adult HIV clinic | 182 | 98.4 |
| Transferred to another health facility | 2 | 1.1 |
| Lost-to-follow-up | 0 | 0.0 |
| Died | 1 | 0.5 |
| Mother’s family planning | ||
| Natural method | 6 | 3.3 |
| Modern method† | 131 | 72.0 |
| Method unspecified | 7 | 3.8 |
| No family planning | 38 | 20.9 |
| Mother on anti-retroviral therapy | ||
| Yes | 182 | 100 |
| Not reported | 0 | 0 |
| Infant outcome | ||
| Completed 18 months follow-up | 172 | 93.0 |
| Transferred to another facility | 8 | 4.3 |
| Lost-to-follow-up | 4 | 2.2 |
| Died | 1 | 0.5 |
| Infant HIV diagnosis | N=176 | |
| Positive | 2 | 1.1 |
| Negative | 166 | 94.3 |
| Indeterminate | 8 | 4.6 |
| Time of final diagnosis if infant HIV negative | N=166 | |
| 15-18 months | 153 | 92.2 |
| 6-14 months | 10 | 6.0 |
| 0-2 months | 3 | 1.8 |
| Infant acute malnutrition†† | N=95 | |
| Severe | 1 | 1.1 |
| Moderate | 3 | 3.2 |
| Mild | 10 | 10.5 |
| No malnutrition | 81 | 85.3 |
| Infant chronic malnutrition‡‡ | N=93 | |
| Severe | 28 | 30.1 |
| Moderate | 26 | 28.0 |
| Mild | 15 | 16.1 |
| No malnutrition | 24 | 25.8 |
4. Discussion
In this study, we report an integration of interventions resulting in high retention in care for mothers with HIV, with 98.4% alive and in care after 18 months post-partum and 100% linkage back to routine adult HIV care following discharge from the Combined Clinic. Few studies have specifically examined retention in care for mothers during the post-partum period, though there are estimates from SSA that 50-82% of HIV-positive mothers remain in ART care in the first six months post-delivery.[6, 18-20] In Rwanda, one recent report estimated a post-partum retention rate of 67% at 11-13 months.[21] Most PMTCT programs in SSA have separate mother and infant services[6, 19, 20] and attempts for service integration are unstructured and often restricted to prenatal, rather than post-partum care.[18] The barriers that mothers face in the prenatal period, such as lack of family or community support, stigma, waiting time at hospitals and transport difficulties[3, 6] are heightened in the post-partum period, particularly with increased clinic visits when mother and infant services are separate. In addition, mothers may prioritize infant care over their own, increasing maternal loss-to-follow-up.[6] Our findings suggest that integration of maternal and infant services in the extended post-partum period and providing additional CBAP supports may promote retention in the post-partum clinic by reducing traditional barriers to care for mothers and facilitating re-linkage to adult paediatric HIV care.
Additionally, mothers in the Combined Clinic had high uptake of FP methods. The rate of FP utilization in this cohort (72.0%) compares favourably to FP utilization among HIV-positive women elsewhere in East Africa, ranging from 58-75%.[22, 23] In Rwanda, FP is routinely offered within HIV services, and previous studies have reported up to 53% of women utilizing FP at any given time.[11, 24] To our knowledge, this is the first study to report rates of FP utilization at 18 months post-partum for mothers with HIV in Rwanda. Our study supports previous literature that integration of FP with HIV care may result in the secondary benefit of increased utilization of FP services. [25]
Retention of infants in this cohort at 15-18 months was considerably higher (93.0%) than for other programs in the region, which have reported retention ranging from 33.0-79.2%,[9, 26] including Rwanda (76.0%).[21] The HIV infection rate among HIV-exposed infants discharged from the Combined Clinic was low (1.1%), and consistent with national reports for MTCT in Rwanda (1.8%);[14] however, the transmission rate in this cohort may be underestimated due to missing results for 18-month tests and indeterminate results without documented confirmatory testing. Overall, rates of MTCT may be lower in Rwanda than in other SSA countries (8.8%)[27] due to wide availability of integrated PMTCT services, high uptake of routine PMTCT services (including prophylaxis and HIV testing), and early implementation of lifelong ART for pregnant women with HIV.[28]
Despite the provision of monthly food supplementation to complement breastfeeding, the nutritional outcomes for infants upon exit from the program were suboptimal. The rate of moderate to severe chronic malnutrition at 15-18 months observed in this cohort is higher than the overall rate of 41.6% reported among children 12-17 months of age in Rwanda.[11] This could be due to the fact that HIV-exposed infants have poorer nutritional status as compared to their non-exposed counterparts, which has been well established in resource-poor settings.[29] In a recent cohort of HIV-exposed infants > 12 months in Rwanda, 63% of males and 37% of females met criteria for moderate to severe chronic malnutrition.[30] However, given that only 51.4% of infants had documented anthropometric measurements at program end, there may be a reporting bias for malnourished children, which could have potentially overestimated the rate of malnutrition in this cohort.
This study had a number of limitations. As the study utilized data routinely collected as part of the clinical care, there were missing data for variables, including FP, infant testing and nutritional outcomes. The incomplete documentation could be due to lack of documentation by treating clinicians with implications such as underestimation of transmission. We recommend review of the patient record system to increase ease and supervision of documentation to ensure complete records. Additionally, as our study did not compare directly to a standard model of care, we cannot directly attribute our findings to the reorganization of care services. Further, additional support provided for patients in this cohort, such as community-based accompaniment and nutritional packages, may have contributed to our findings, and the impact of each component should be studied in the future.
5. Conclusions and Global Health Implications
In summary, we believe these findings suggest that integration of maternal HIV and infant PMTCT services in conjunction with a community-based accompaniment program contributes to high maternal retention in HIV care and treatment following delivery and the breastfeeding period. To our knowledge, this study is the first such program described in the literature, and the favourable utilization and clinical outcomes for both the infant and mother may inform similar programs in related contexts. Although not yet studied in our context, the integration of these services may also provide valuable efficiencies not only for the patient experience, but also for health system organization, efficiency, and financing.[21] Further data on the impact of this model on frequency of patient visits, patient and provider satisfaction, and long-term clinical outcomes may also prove valuable as validation of this service integration to inform future HIV and PMTCT programming in SSA.
Acknowledgement
The authors thank Christine Warugaba, Naomi Nyirahabimana, Nadine Karema and Eline Uwitonze for administrative support and Catherine Kirk and Marie Paul Nisingizwe for assistance with data analysis. We would also like to thank Stephanie Bazubagira and Pascal Nkezabera for their assistance with data collection. This study was developed under the Partners in Health/Inshuti Mu Buzima Operation Research Training Program, developed and facilitated by Bethany Hedt-Gauthier and Neil Gupta.
Conflict of interest: The authors declare that they have no competing interests.
Ethical Approval: Study was approved by an approved Institutional Review or Ethics Board.
Funding: The authors acknowledge the Doris Duke Charitable Foundation African Health Initiative and Partners in Health/Inshuti Mu Buzima Innovation Grants for the financial support of this work.
REFERENCES
- 2015. How AIDS Changed Everything. MDG 6: 15 years, 15 lessons of hope from the AIDS response [Internet]. UNAIDS. Available from: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf
- Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+approach. Current Opinion in HIV and AIDS. 2013;8(5):474-89.
- [Google Scholar]
- Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. Journal of the International AIDS Society. 2013;19; 16(1)
- [Google Scholar]
- Missed opportunities: poor linkage into ongoing care for HIV-positive pregnant women in Mwanza, Tanzania. PLoS One. 2012;9;7(7):e40091.
- [Google Scholar]
- Loss to followup: a major challenge to successful implementation of prevention of mother-to-child transmission of HIV-1 programs in sub-Saharan Africa. International Scholarly Research Notices AIDS. 2012;31:2012.
- [Google Scholar]
- “What They Wanted Was to Give Birth;Nothing Else”: Barriers to Retention in Option B+Care Among Postpartum Women in South Africa. Journal of Acquired Immune Deficiency Syndrome. 2014;67:e12-18.
- [Google Scholar]
- The contribution of HIV to pregnancy-related mortality: a systematic review and meta-analysis. AIDS. 2013;19;27(10):1631-9.
- [Google Scholar]
- Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. Journal of Acquired Immune Deficiency Syndrome. 2011;56(4):e104.
- [Google Scholar]
- The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;13;27(17):2787-97.
- [Google Scholar]
- Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. Journal of Acquired Immune Deficiency Syndrome. 2011;56(5):e122.
- [Google Scholar]
- 2016. Rwanda Demographic and Health Survey 2014-15 – Final report [Internet]. Demographic Health Survey. Available from: http://www.statistics.gov.rw/publication/demographic-and-health-survey-20142015-final-report
- 2014. Health Sector Annual Report July 2013-June 2014 [Internet]. Rwanda Ministry of Health. Available from: http://www.moh.gov.rw/fileadmin/templates/MOH-Reports/HEALTH_SECTOR_ANNUAL_REPORT_July_2013-June_2014.pdf
- 2010. Effectiveness of National Program for the Prevention of Mother-to-Child Transmission (PMTCT) of HIV in Rwanda 2010 [Internet]. TracPlus Report. Available from: http://www.sph.ur.ac.rw/IMG/pdf/Effectiveness_of_National_Program_for_the_Prevention_of_Mother-to-Child_Transmission_PMTCT_of_HIV_in_Rwanda_May_2010.pdf
- 2015. Global HIV/AIDS: Rwanda [Internet]. CDC. Available from: http://www.cdc.gov/globalaids/global-hiv-aids-at-cdc/countries/rwanda/
- Scaling up early infant diagnosis of HIV in Rwanda 2008–2010. Journal of Public Health Policy. 2013;1;34(1):2-16.
- [Google Scholar]
- Clinical Outcomes of a Comprehensive Integrated Program for HIV-Exposed Infants: A 3-Year Experience Promoting HIV-Free Survival in Rural Rwanda. Journal of Acquired Immune Deficiency Syndrome. 2013;1;62(4):e109-14.
- [Google Scholar]
- 2011. Zscore06: Stata command for the calculation of anthropometric z-scores using the 2006 WHO child growth standards [Internet]. International Food Policy Research Institute. Available from: http://www.ifpri.org/staffprofile/jef-leroy
- Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. Journal of the International AIDS Society. 2014;10;17(1)
- [Google Scholar]
- Retention in care under universal antiretroviral therapy for HIV infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS. 2014;20;28(4):589.
- [Google Scholar]
- Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+PMTCT programme in Lilongwe, Malawi. Tropical Medicine and International Health. 2014;19(11):1360-1366.
- [Google Scholar]
- A secondary analysis of retention across the PMTCT cascade in selected countries: Rwanda, Malawi, Kenya and Swaziland [Internet] 2015. Population Council. Available from: https://popcouncil.org/research/a-secondary-analysis-of-retention-across-the-pmtct-cascade-in-selected-coun
- [Google Scholar]
- Uptake of family planning methods and unplanned pregnancies among HIV-infected individuals: a cross-sectional survey among clients at HIV clinics in Uganda. Journal of the International AIDS Society. 2011;14(1):1.
- [Google Scholar]
- Contraceptive use and method preference among HIV positive women in Addis Ababa, Ethiopia: a cross sectional survey. BMC Public Health. 2014;14:566.
- [Google Scholar]
- Improved access increases postpartum uptake of contraceptive implants among HIV-positive women in Rwanda. The European Journal of Contraception & Reproductive Health Care. 2009;14(6):420-5.
- [Google Scholar]
- Integration of family planning services into HIV care and treatment in Kenya: a cluster-randomized trial. AIDS. 2013;27(Suppl 1):S77-85.
- [Google Scholar]
- Reducing mother-to-child transmission of HIV: findings from an early infant diagnosis program in south-south region of Nigeria. BMC Public Health. 2012;12(1):1.
- [Google Scholar]
- Evaluating the impact of Zimbabwe’s prevention of mother to child HIV transmission program: population-level estimates of HIV-free infant survival pre-option A. PLoS One. 2015;10(8):e134571.
- [Google Scholar]
- HIV-free survival among nine-to 24-month-old children born to HIV-positive mothers in the Rwandan national PMTCT programme: a community-based household survey. Journal of the International AIDS Society. 2012;15(1):1.
- [Google Scholar]
- Impact of HIV exposure on health outcomes in HIV-negative infants born to HIV-positive mothers in Sub-Saharan Africa. Journal of Acquired Immune Deficiency Syndrome. 2014;65(2):182-9.
- [Google Scholar]
- Sex differences in nutritional status of HIV-exposed children in Rwanda: a longitudinal study. Tropical Medicine & International Health. 2015;20(1):17-23.
- [Google Scholar]





