Translate this page into:
A Tale of Two Medicines: The Need for Ownership, End-to-End Planning and Execution for Development and Introduction of Maternal Health Medicines

* Corresponding author: Sara Rushwan, Concept Foundation, Avenue de Sécheron, Geneva, Switzerland. Tel: +41 22 734 2560 s.rushwan@conceptfoundation.org
-
Received: ,
Accepted: ,
How to cite this article: Gülmezoglu M, Chinery L, Rushwan S, Ammerdorffer A. A tale of two medicines: The need for ownership, end-to-end planning and execution for development and introduction of maternal health medicines. Int J MCH AIDS. 2024;13:S9-14. doi: 10.25259/IJMA_21_2024
Abstract
Postpartum hemorrhage (PPH) persists as the leading direct cause of maternal mortality in low- and middle-income countries (LMICs) and is a major global health challenge. Following favorable evidence from pivotal efficacy clinical trials, the World Health Organization (WHO) recommends the use of heat-stable carbetocin to prevent PPH as a viable substitute in settings where maintaining a cold chain for thermosensitive uterotonics is compromised, and tranexamic acid as an adjunct therapy for PPH treatment. However, the implementation of these drugs has been hindered by several challenges, such as decentralized and disorganized procurement, poor quality assurance, inadequate supply chain management, and limited access in many LMICs.
While including maternal health drugs in the essential medicines list and adopting updated global recommendations are necessary steps forward, they are not enough to guarantee access unless there is end-to-end (E2E) thinking, planning, and execution for essential maternal health commodities. We describe distinct access challenges between the two drugs, both having compelling safety and efficacy data and normative recommendations around the same time; one patent protected and owned by a pharmaceutical company and another with multiple generic manufacturers. We highlight the need for coordinated action to facilitate access to evidence-based maternal health commodities.
Keywords
Postpartum Hemorrhage
Heat-Stable Carbetocin
Tranexamic Acid
End-to-End Planning
Maternal Mortality
Maternal Health
Market Access
INTRODUCTION
Postpartum hemorrhage (PPH), defined as bleeding of 500 mL or more within 24 hours of childbirth, accounts for 20% of maternal deaths globally and mostly in low- and middle-income countries (LMICs). Obstetric hemorrhage accounts for 27% of global maternal mortality, of which more than two-thirds is attributed to PPH.[1] Administering prophylactic uterotonics can potentially reduce PPH deaths by half and is a prioritized intervention, with oxytocin being the most widely used. However, the magnitude of the burden necessitates accelerating the advancement of innovative interventions.[2] In 2012, Merck Sharpe & Dohme (MSD) for Mothers and Ferring Pharmaceuticals engaged the Human Reproduction Program/World Health Organization (HRP/WHO) regarding a new formulation of carbetocin (a synthetic analog of oxytocin that is stable at room temperature) and presented to WHO the stability data. From that initial contact, the project Carbetocin Hemorrhage Prevention (CHAMPION) was conceived. A pivotal randomized, double-blind, non-inferiority trial was implemented comparing intramuscular injection of heat-stable carbetocin (HSC) with oxytocin after vaginal birth, to evaluate the effects of HSC on the prevention of PPH and a concrete, sustained market access plan.[3–5]
Almost concurrently, the World Maternal Antifibrinolytic (WOMAN) trial was conceived by the London School of Hygiene and Tropical Medicine following the data generated by their earlier trial showing a strong beneficial effect of tranexamic acid (TXA) on improving traumatic hemorrhage outcomes.[6] This randomized, double-blind, placebo-controlled trial assessed the effects of early administration of TXA, an antifibrinolytic agent, as an adjunct therapeutic for PPH treatment, with its first meeting taking place in October 2012.[7]
Conducted between 2012 and 2018, the CHAMPION and WOMAN trials encountered organizational and implementation challenges. Project CHAMPION was conducted as a Good Clinical Practice (GCP)-compliant, regulatory-powered, pivotal phase III trial involving 10 countries and nearly 30,000 women, making it the largest PPH prevention trial. The WOMAN trial was, at the time, the largest PPH treatment trial recruiting more than 20,000 women in 21 countries. The results of both trials were published in prestigious journals[4,7] and WHO recommendations were updated accordingly in 2017 (to include TXA for PPH treatment) and in 2018 (to include HSC for PPH prevention), as well as the essential medicines list (EML), without delay.[8–10]
Since 2018, progress in the access pathways for the two drugs has differed significantly. In this paper, we argue that there are significant challenges that lead to delayed access to medicines when there is no timely end-to-end (E2E) access planning and execution. This lack of planning is widespread for maternal health medicines because most are generic and re-purposed for maternal health indications without a committed “owner”.[11] The implications of the lack of committed ownership are market challenges discouraging pharmaceutical companies from investing in this area, leading to a chaotic maternal health research field.
END-TO-END PLANNING AND EXECUTION FOR END-USER ACCESS
An E2E approach to planning and execution for new medicines to move from research to bedside access is critical. The E2E thinking is a standard process within the pharmaceutical industry where the objective is to make the medicine accessible to the whole market of interest as fast as possible so that more patients can benefit from the innovation, and the return on investment can be realized and maximized in a timely manner. The pharmaceutical industry commits considerable resources to develop and execute its “launch excellence” plans – from clinical trial design to regulatory approval to medical/patient education to peak bedside access – to maximize the potential of its innovations. A summary of the E2E approach is illustrated in Figure 1.
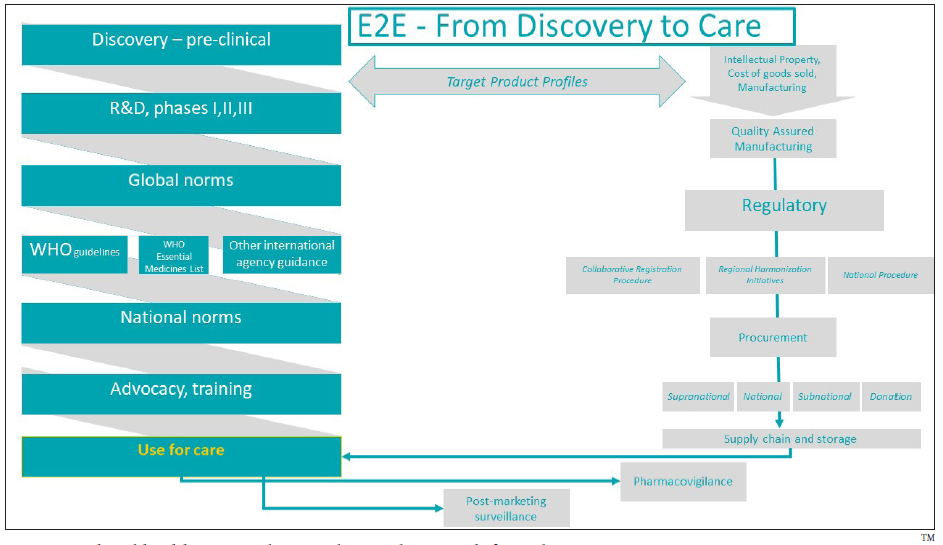
- Pharmaceutical and health commodities end-to-end approach from discovery to care.
In maternal health, most medicines are re-purposed, relatively old, and off-patent, with few pharmaceutical industry-owned and developed medicines – examples include aspirin, magnesium sulfate, and nifedipine.[12] One might think that an off-patent, repurposed medicine could be more readily accessible. However, the experience suggests that it is not certain.
Issues to Consider from a Lack of End-to-End Planning for Maternal Health Medicines
Without a structured E2E approach, medicine access plans can become disorganized and inconsistent, leaving many patients unable to benefit from the intervention. There are bottlenecks at the research and market access levels that impede the discovery-to-care pathway. In maternal health, research is largely conducted within academia and without specific ownership or interest by companies to provide access to LMICs, often without any substantive access planning. Another issue is the lower purchasing power of LMICs, which impacts governments’ willingness to procure medicines based on affordability, and this heavily influences market access, uptake, and use of maternal health innovations. The LMIC context should be considered from the early discovery phase to ensure that target populations in LMICs have access to innovations.[13]
Market challenges have broadly impacted the development of maternal health medicines, as investment in maternal health has been marginalized in research and development (R&D) business models due to perceived financial risks. One of the barriers in R&D is the difficulty in recruiting pregnant women for clinical trials. A recently published comprehensive mixed-methods systematic review revealed a wide range of evidence of the factors that hinder or facilitate the inclusion of pregnant women in clinical trials.[14] Other issues surface during the registration phase due to challenges in obtaining stringent regulatory product approval. Once the product is in the market, bottlenecks include policy inclusion, establishing sustainable procurement pathways, and medicine pricing influencing uptake.[15]
Applying an End-to-end Lens to the Introduction of Heat-stable Carbetocin and Tranexamic Acid
Considering the E2E domains in Figure 1, there are similarities and differences between HSC and TXA after more than 10 years of efforts in the global maternal health space to introduce and scale-up the use of these medicines. It is noteworthy that the similarities are more downstream at the level of global norms and recommendations, national policy inclusion, innovation introduction, training, supply chain/storage needs, and international procurement. Meanwhile, the differences between the two medicines are more upstream related to the discovery, R&D, manufacturing, price, and quality assurance. The similarities and differences per domain are presented in Table 1.
| End-to-end (E2E) domain | Heat-stable carbetocin (HSC) | Tranexamic acid (TXA) |
|---|---|---|
| Discovery, research, and development |
|
|
| Ownership, manufacturing, and price |
|
|
| Quality assurance |
|
|
| Global norms and recommendations |
|
|
| Regulatory strategy and approvals |
|
|
| International procurement | Included in UNFPA catalog. | Included in UNFPA catalog. |
| Supply chain/storage needs | Heat stable, no specific concerns. | Heat stable, no specific concerns. |
| National policy inclusion |
|
|
| Access considerations |
|
|
| Training | Training packages available, used in pilot studies with success. | Training focused on timely use and in a bundle. Used in pilot studies with success. |
| Innovation introduction | Several grant-funded implementation projects in SSA and India. | Several grant-funded implementation projects in SSA. |
| Pharmacovigilance/safety |
|
|
EML: Essential medicines list, EOI: Expression of interest, IDA: IDA foundation, IP: Intellectual property, LMICs: Low-and-middle-income countries, LSHTM: London School of Hygiene and Tropical Medicine, MMR: Maternal mortality ratio, PPH: Postpartum hemorrhage, PQ: Prequalification, QA: Quality assured, SRA: Stringent regulatory authority, SSA: Sub-Saharan Africa, UNFPA: United Nations Population Fund, WHO: World Health Organization.
Additionally, there are some key differences between HSC and TXA in terms of ownership, regulatory strategy, and approvals (summarized in Table 1) that subsequently delay access in countries where the PPH burden is highest. Despite the publication of an expression of interest for WHO prequalification in 2018, no pharmaceutical company to date has come forward and applied for prequalifying a TXA product (as of August 2024). HSC, on the other hand, despite having the Swissmedic approval, has been WHO-prequalified as part of the innovator’s broader quality, regulatory, and access strategies, which is the first step to accelerate access through in-country registration of the medicine, update of national policies, and qualifying for inclusion in UNFPA’s procurement catalog. This provides a channel for quality-assured public sector procurement of this medicine, and HSC has been gradually registered and introduced in several LMICs since the trial. Concerted efforts remain for wide-scale registration of the drug in LMICs.
Until a recent study indicated quality concerns with TXA products,[16] there were no systematic efforts to assess and understand whether TXA for intravenous injections had quality issues, despite TXA having so many life-saving indications. This means that despite TXA being an inexpensive and effective drug that reduces many forms of bleeding, the lack of prequalified TXA products (specifically for PPH treatment) necessitates a stringent quality-assurance process for TXA products which have multiple indications and a large use case in LMICs.
Most of the TXA work after the WHO recommendation and EML inclusion focused on implementation research and barriers and facilitator assessments. In our opinion, the lack of a “product owner” leads to a patchy implementation that often does not have an E2E approach. The benefits of product ownership include the development of PPH-specific medicines that have been adequately tested, thereby fostering greater global investment into innovative fit-for-purpose medicines that are appropriate for low-resource settings. Product ownership facilitates registration which is an important step in the pathway to in-country product introduction and access.
To address the need for national policy updates to trigger the required in-country processes to accelerate access to these medicines, Concept Foundation, WACI Health, the International Federation of Gynecology and Obstetrics (FIGO), and the International Confederation of Midwives (ICM) worked with Ministries of Health, civil society organizations, and professional associations to facilitate the update of national policies in sub-Saharan African countries between 2019 and 2022.[19] Notwithstanding the shortcomings of not utilizing E2E thinking to bridge the gap between policy and access, such initiatives can accelerate policy change and the adoption of updated WHO recommendations at the national level.
The differences identified in Table 1 are indicative of a lack of E2E alignment and coordination within the maternal health community. However, there is growing momentum for a collective E2E-driven approach, such as the recently launched PPH roadmap which is a multi-pronged strategy with key priorities for research, advocacy, and implementation to drive progress towards meeting the 2030 Sustainable Development Goals.[20]
CONCLUSION AND GLOBAL HEALTH IMPLICATIONS
Despite the potential of HSC and TXA to address quality barriers to PPH care, the field lacks a holistic E2E approach that robustly aligns normative change, market preparation, product registration, introduction, implementation, and scale-up. The challenges outlined significantly delay market access and are specifically critical for maternal health medicines, where decentralized and disorganized procurement, poor quality assurance, weak supply chains, and overall fragmentation are prevalent. What we have described in this paper is not new. Since most maternal health medicines in use, such as nifedipine, magnesium sulfate, hydralazine, and misoprostol, are re-purposed generics, they face challenges similar to those encountered with TXA access. Without any public or private sector sponsor, these quality and other access issues will not be eliminated in the foreseeable future.
Substantive work has been done to improve these challenges several years ago with the United Nations Commission for Life-Saving Commodities between 2012 and 2015, including updating EMLs and introducing post-marketing quality surveillance for reproductive and maternal health drugs.[21] As new medicines and devices enter maternal health care, similar initiatives and sustained efforts for quality assurance and overall E2E thinking are needed. E2E thinking, planning, and execution for access to essential maternal health medicines should not be restricted to private sector-owned commodities, but broadened to attain universal health coverage which demands cohesive vertical scale-up for sustainability. It can take years from commodity introduction to scale within the public health sector of LMICs. Takeaways from the introduction of HSC and TXA should serve as valuable lessons for cultivating a paradigm shift that mainstreams E2E planning to diminish the equity gap to access high-impact maternal health interventions.
Key Messages
-
Heat-stable carbetocin and tranexamic are recommended evidence-based interventions for postpartum hemorrhage with similarities and differences in their introduction and access pathways.
-
The maternal health field lacks an all-encompassing end-to-end approach which factors in the low-and-middle-income country context.
-
Product ownership is key to a structured end-to-end approach for the development, introduction, and access to maternal health medicines.
Acknowledgments
None.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest
Concept Foundation assists the innovator of heat-stable carbetocin in registering the medicine in low- and middle-income countries. Concept Foundation works with one tranexamic acid innovator to develop products that are easier to use at peripheral levels of health care.
Financial Disclosure
Nothing to declare.
Funding/Support
This Special Collection was supported by funding from MSD, through its MSD for Mothers initiative and is the sole responsibility of the authors. MSD for Mothers is an initiative of Merck & Co., Inc., Rahway, NJ, USA.
Ethics Approval
Not applicable.
Declaration of Patient Consent
Not applicable.
Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
None.
Special Collection
This article is published as part of the special collection on prevention and treatment of postpartum hemorrhage in high-burden low- and middle-income countries: building cross-national evidence through implementation research.
REFERENCES
- Global causes of maternal death: A WHO systematic analysis. Lancet Glob Health.. 2014;2(6):323-33. https://doi.org/10.1016/s2214-109x(14)70227-x
- [Google Scholar]
- Uterotonics for prevention of postpartum haemorrhage: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth.. 2021;21(Suppl 1):230. https://doi.org/ 10.1186/s12884-020-03420-x
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally: Study protocol for a randomized controlled trial. Trials.. 2016;17(1):143. doi:10.1186/s13063-016-1271-y
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med.. 2018;379(8):743-52. doi: 10.1056/nejmoa1805489
- [CrossRef] [PubMed] [Google Scholar]
- Advancing a new drug to improve global maternal health through a tripartite initiative. Harvard Business Publishing; 2020. [Accessed 2024 Jan 16]. Available from: https://www.globalhealthdelivery.org/publications/advancing-new-drug-improve-global-maternal-health-through-tripartite-initiative
- Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet.. 2010;376:23-32. doi:10.1016/S0140- 6736(10)60835-5
- [CrossRef] [PubMed] [Google Scholar]
- Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet.. 2017;389(10084):2105-16. doi:10.1016/S0140-6736(17)30638-4
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage. Geneva: World Health Organization; 2017. [Accessed 2024 Apr 15]. https://www.who.int/publications/i/item/9789241550154
- WHO recommendations: Uterotonics for the prevention of postpartum haemorrhage. Geneva: World Health Organization; 2018. [Accessed 2024 Apr 15]. Available from: https://apps.who.int/iris/handle/10665/277276
- WHO model list of essential medicines – 21st list, 2019. Geneva: WHO; 2019. [Accessed 2023 Nov 24]. Available from: https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06
- “End-to-end thinking” is paving the way to future medicines. Published 2019. [Accessed 2024 Mar 16]. Available from: https://www.fip.org/end-to-end-thinking-is-paving-the-way-to-future-medicines
- The drug drought in maternal health: an ongoing predicament. Lancet Glob Health.. 2024;12(7):e1174-83. doi: 10.1016/S2214-109X(24)00144-X
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Medicines for pregnancy-specific conditions – Research, development, and market analysis. Published 2021. [Accessed 2024 Jan 16]. Available from: https://www.conceptfoundation.org/wp-content/uploads/2021/06/AIM-Medicines-for-Pregnancy-Specific-Conditions-Research-Development-and-Market-Analysis.-Excl-interv.-names.pdf
- Factors influencing the participation of pregnant and lactating women in clinical trials: A mixed-methods systematic review. PLoS Med.. 2024;21(5):e1004405. https://doi.org/10.1371/journal. pmed.1004405
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Concept Foundation. Market Challenges and Potential Solutions for the Development and Introduction of Medicines for Pregnancy Specific Conditions. Published 2021. [Accessed 2024 Apr 16]. Available from: https://www.conceptfoundation.org/wp-content/uploads/2023/10/Del.-1.2-Maternal-health-medicines-market-16.04.21.pdf
- Quality of oxytocin and tranexamic acid for the prevention and treatment of postpartum hemorrhage in Kenya, Nigeria, South Africa, and Tanzania. Int J Gynaecol Obstet.. 2022;158(Suppl 1):46-55. doi:10.1002/ijgo.14197
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tranexamic acid at cesarean delivery: Drug-error deaths. BJOG.. 2023;130(1):114-17. doi: 10.1111/1471-0528.17292
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The compatibility of oxytocin and tranexamic acid injection products when mixed for co-administration by infusion for the treatment of postpartum haemorrhage: An in vitro investigation. BJOG.. 2023;130(7):741-9. doi: 10.1111/1471-0528.17398
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in updating national guidelines and essential medicines lists in Sub-Saharan African countries to include WHO-recommended postpartum hemorrhage medicines. Int J Gynaecol Obstet.. 2022;158(Suppl 1)Int J Gynaecol Obstet.. 2022;158(Suppl 1):11-13. doi:10.1002/ijgo.14269
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A roadmap to combat postpartum haemorrhage between 2023 and 2030. Geneva: World Health Organization; 2023. [Accessed 2024 Apr 8]. Available from: https://www.who.int/publications/i/item/9789240081802
- The UN Commission on life saving commodities 3 years on: Global progress update and results of a multicountry assessment. Lancet Glob Health.. 2016;4(4):e276-86. doi:10.1016/S2214-109X(16)00046-2
- [CrossRef] [PubMed] [Google Scholar]





