Translate this page into:
Diagnosis, Treatment and Outcome of Gestational Trophoblastic Neoplasia in a Low Resource Income Country
*Corresponding author email: mamourgueyeobgyn@yahoo.fr
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background and Introduction:
Gestational trophoblastic disease (GTD) is a disease of the proliferative trophoblastic allograft. Diagnosis and treatment of GTN in low resource-income countries is challenging due to numerous factors. The objective of this study was to review outcomes of gestational trophoblastic neoplasia in women of low socioeconomic status with limited resources and social support.
Methods:
This study was performed at Gynecologic and Obstetric Clinic of Dakar Teaching Hospital, the reference Centre of Gestational trophoblastic diseases in Senegal from 2006 to 2015.
Results:
Out of 1088 patients followed for gestational trophoblastic disease during the study period, 108 patients were diagnosed and treated for GTN: 88 low-risk and 20 high-risk. Low-risk patients received an average of 6.9 cycles of initial single-agent chemotherapy. Twelve patients had persistent disease and were switched to a second line multi-agent chemotherapy. Finally 94.3% of low-risk patients achieved remission. All high-risk patients were initially treated with multi-agent chemotherapy, averaging 7 cycles. Five of the eighty-eight low-risk patients and twelve of the 20 high-risk patients died of disease.
Conclusion and Global Health Implications:
Early adequate treatment ensures an excellent prognosis for patients with GTN. In low-income countries, difficulties encountered in diagnosis and treatments worsen the prognosis of GTN patients. Clinical trials are needed to find out affordable schedules or drugs for a better treatment.
Keywords
Gestational Trophoblastic Neoplasia
Multi-agent Chemotherapy
Methotrexate
1. Introduction
The gestational trophoblastic disease (GTD) is a disease of the proliferative trophoblastic allograft.[1] After uterine evacuation, 80% of hydatiform mole cases follow spontaneous remission, but in about 20% of cases, trophoblastic tissue escapes maternal immunological surveillance, invades the maternal host, and progresses to post molar gestational trophoblastic neoplasm (GTN).[2] In 2000, the International Federation of Gynecology and Obstetrics (FIGO) and the World Health Organization (WHO) combined anatomic staging with a modification of the WHO prognostic index score, for an internationally agreed risk assessment and treatment approach (Tables 1 and 2).[1] While in the traditional WHO scoring system the GTN was divided into low-, medium- and high-risk groups, in the new system the GTN is stratified into two groups: (i) low-risk group (score ≤6) intimating single-agent chemotherapy; and (ii) high-risk group (score >6) mandating multi-agent chemotherapy.[1] Risk is defined as the risk of developing drug resistance (Methotrexate or MTX) as determined by the WHO Prognostic Scoring System. All patients with non-metastatic disease and patients with risk scores <7 are considered to have low-risk disease. Patient with score > 6 are considered to have high-risk disease.
| Stage I | Disease confined to the uterus |
| Stage II | GTN extends outside of the uterus, but is limited to the genital structures (adnexa, vagina, broad ligament) |
| Stage III | GTN extends to the lungs, with or without known genital tract involvement |
| Stage IV | All other metastatic sites |
| Scores | 0 | 1 | 2 | 4 |
|---|---|---|---|---|
| Age (years) | <40 | ≥40 | ||
| Antecedent pregnancy | Mole | Abortion | Term | |
| Antecedent pregnancy from index pregnancy | <4 | 4-6 | 7-12 | ≥13 |
| Pre-treatment serum hCG (UI/ml) | <103 | 103-<104 | 104-<105 | ≥105 |
| Largest tumor size (including uterus) | 3-<5 cm | ≥5 cm | ||
| Site of metastasis | Lung | Spleen, kidney | Gastro-intestinal | Liver, Brain |
| Number of metastases | 0 | 1-4 | 5-8 | >8 |
| Previous failed chemotherapy | Single drug | 2 or more drugs | ||
The measurement of human chorionic gonadotropin provides an accurate and reliable tumor marker for diagnosis, monitoring the effects of chemotherapy and follow-up to determine recurrence.[2] Diagnosis and treatment of GTN in low resource-income countries is challenging due to numerous factors. The objective of this study was to review outcomes of gestational trophoblastic neoplasia in women of low socioeconomic status with limited resources and social support.
2. Methods
This was a retrospective cross-sectional study performed at Gynecologic and Obstetric Clinic of Dakar Teaching Hospital, the reference Center of GTD in Senegal. Two protocols of follow up were used in our center in two points of time. From 2006 to 2010, women were followed-up in accordance with hospital protocol called “Score de Dakar”.[3] This score includes age, parity, area of residence, blood group, revenue, level of post-evacuation hCG, character of vesicles, duration of pregnancy and history of hydatiform mole. According to this score, patients were classified into low (LR), medium (MR) and high risk (HR) of progression to GTN. For the LR, monitoring of hCG is recommended, for MR, single agent chemotherapy (Chem-P) with methotrexate (25mg per day from D1 to D5) is prescribed and for HR patients, hysterectomy associated to chemotherapy (Chem-T) (methotrexate 20mg/Kg i.m and cyclophosphamide 500mg i.v: day 1 and day 8 every 3 weeks) is recommended until achieving undetectable serum hCG.
At the beginning of 2011, patients were followed according to FIGO’s recommendations.
We follow, in our department, women of low socioeconomic status with limited resources and social support. As it was difficult in our setting to implement weekly β-hCG test, we decided to proceed as follows: 1) if initial hCG measurement done at least 3 weeks after uterine evacuation was < 100 000 IU/L without any evidence of choriocarcinoma, the next measurement was prescribed 4 weeks later; 2) if initial hCG measurement done at least 3 weeks after uterine evacuation was ≥ 100 000 IU/L or any evidence of choriocarnima, the next measurement was prescribed 1 or 2 weeks later depending on financial means of patients. In Senegal, HCG measurement costs between $20 US and $50 US. Based on the above clinical determinations, our department staff decided to use slightly modified FIGO 2000 criteria as follow:
-
A high β-hCG level that has remained stable for 2 consecutive tests in 4 weeks,
-
A 10% increase in β-hCG level for 2 consecutive tests in 2 weeks,
-
Any elevation in β-hCG level for 6 months after evacuation, and
-
A histopathologic diagnosis of choriocarcinoma.
Staging and scoring GTN patients were done according to Table 1 (FIGO staging system) and Table 2 (WHO scoring system).[1] Patients of FIGO score <7 were considered to be low-risk. Treatment consisted of methotrexate (MTX) based on the 8-day protocol consisting of 1 mg/kg MTX in combination with 0.1mg/kg folinic acid (FA) every other day or MTX combined to cyclophosphamide. In low-risk gestational trophoblastic neoplasia (LRGTN), stable or increasing β-hCG levels after two courses of treatment was considered a failure to initial chemotherapy. Patients were switched to a combination therapy: EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, oncovin); MAC (methotrexate, actinomycin D, cyclophosphamide) or any other combination according to availability of drugs and financial means of patients. Patients of FIGO score up or equal to 7 were considered to be high-risk. In high-risk gestational trophoblastic neoplasia (HRGTN), stable or increasing β-hCG levels after two courses of treatment was considered a failure to the initial multi-agent chemotherapy. Patients were switched to another combination therapy according to availability of drugs and means of patients. Statistical Package for Social Science, Version 20.0 was used for all statistical analyses. In the following, we report data for 108 patients diagnosed for GTN out of 1088 patients followed for gestational trophoblastic disease.
3. Results
Out of 1,088 patients followed for trophoblastic diseases, one hundred and eight patients were diagnosed and treated for GTN: 88 low-risk and 20 high-risk. The income of the couple appreciated on their profession and their husbands found 68.5% low income. Indeed, 69.4 % of patients were without occupation. The others were working in low paid occupations (salesperson, small trade.). In low-risk patients, single agent-chemotherapy with Methotrexate was used in 83 patients while Methotrexate and Cyclophosphamide were used in 5 patients as intimated Dakar’s protocol before 2011. Mean number of cycles required to achieve complete response was 6.9 which included administration of two additional cycles past initial normalization of β hCG titers (range 2-23 cycles). Twelve patients had persistent disease and were switched to a second line multi-agent chemotherapy: Etoposide-Vincristine-Cyclophosphamide (3 patients), 5FU-MTX-Etoposide (3 patients), Etoposide-Methotrexate-ActD-Cyclophosphamide-Vincritsine (EMACO) (3 patients), Methotrexate-Act-D-Cyclophosphamide (3 patients). Seven patients achieved remission after second line chemotherapy. Finally 94.3% of low-risk patients achieved remission. Patient’s characteristics are summarized in Table 3.
| Variables | Low risk gestational trophoblastic neoplasia | High risk gestational trophoblastic neoplasia | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Age range (years) | ||||
| <40 | 69 | 78.4 | 17 | 85 |
| ≥40 | 19 | 21.6 | 3 | 15 |
| Time to GTN (weeks) | ||||
| < 16 | 55 | 62.5 | 4 | 20 |
| 16-24 | 13 | 14.7 | 2 | 10 |
| 25-52 | 18 | 20.5 | 5 | 25 |
| > 52 | 2 | 2.3 | 9 | 45 |
| FIGO stage | ||||
| I | 82 | 93.2 | 6 | 30 |
| II | 4 | 4.5 | 3 | 15 |
| III | 2 | 2.3 | 7 | 35 |
| IV | - | - | 4 | 20 |
| Pre-treatment serum hCG level (UI/ml) | ||||
| < 100 000 | 79 | 89.8 | 10 | 50 |
| ≥ 100000 | 9 | 10.2 | 10 | 50 |
| Surgery (Hysterectomy) | ||||
| Yes | 10 | 11.4 | 10 | 50 |
| No | 78 | 88.6 | 10 | 50 |
| Chemotherapy (1st line) | ||||
| Methotrexate | 83 | 94.3 | 7 | |
| Methotrexate-C | 5 | 5.7 | 5 | |
| Methotrexate-Etoposide | - | |||
| 5FU-MTX-VP16 | - | 2 | ||
| EMACO | - | 4 | ||
| MTX-VP16 | 2 | |||
| Outcome | ||||
| Remission | 83 | 94.3 | 8 | 40 |
| Death | 5 | 5.7 | 12 | 60 |
C: Cyclophosphamide 5FU: Fluorouracile MTX: Metothrexate VP16: Etoposide
High-risk patients were initially treated with either single-agent or multi-agent chemotherapy depending on their financial means, averaging 7 cycles which included administration of two additional cycles past initial normalization of βhCG titers. It consisted of single agent chemotherapy with Methotrexate (7 patients), Methotrexate-Cyclophosphamide (5 patients), 5FU-Methotrexate-Etoposide (2 patients), MTX-Etoposide (2 patients) and EMACO (2 patients). Eight patients achieved remission. Five of the eighty-eight low-risk patients and twelve of the 20 high-risk patients died of disease. As they experienced resistance, multi-agent chemotherapy was prescribed. Unable to achieve the chemotherapy drugs appropriate to their condition (MAC or EMACO), they gave up and returned to village. After a couple of weeks, there were readmitted with visceral metastases and deceased in the following days. The average survival time was 9.9 months ([95% CI] 5.369-14.429) and the median was 2.87 months ([95% CI] 0.000-14.982) as shown in Figure 1.
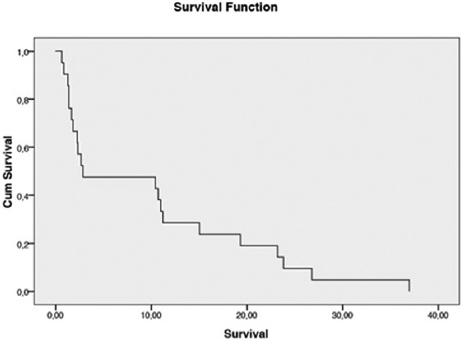
- Survival curve using Kaplan-Meier method
- Mean: 9,89 months IC 95% (5,369-14,429); Median: 2,87 months IC 95% (0,000-14,982)
4. Discussion
4.1. Treatment and outcome of low-risk gestational trophoblastic neoplasia (LRGTN)
Extensive experience has been accumulated in treating low-risk GTN over time, and over 14 different types of chemotherapy regimens have been described, but no consensus has been reached about the preferred first-line treatment. As there is no strong evidence to confirm the superiority of any one method, several treatments have been arbitrarily used in different centers. However, a consensus has been reached about the use of a single agent, such as Methotrexate (MTX) or Act D, for patients with low-risk disease. These drugs have induction remission rates of 50 to 90%.[4] Primary response variability results from differences in drug doses, times and administration modes, as well as patient selection. In general, weekly IM or intermittent IV infusion of MTX and biweekly Act D are less effective than MTX and Act D for five days or MTX/FA (FA for folinic acid) for eight days. However, almost all patients are cured and have their fertility preserved despite the differences in initial remission after primary chemotherapy.[4] The weekly MTX 30–50 mg/m2 regimen has the advantage of convenience and low cost and toxicity, but has the lowest complete response rate among all other regimens.[4] With Methotrexate associated or not to folinic acid for eight days, rate of response was near 95% in our study. In the Cochrane review, Alazzam et al. aimed to determine the efficacy and safety of first line chemotherapy in the treatment of low-risk GTN. The review included randomized controlled trials (RCTs), quasi-RCTs and non-RCTs (cohort and case control studies (CCS)) for the treatment of low risk GTN. Eight studies met the review entry criteria (n = 769). Based on the available evidence from the included RCTs, the authors conclude that “pulsed” dactinomycin is superior to weekly parenteral methotrexate at the reported dosages. However, the authors believe that rigorously designed, multicentred, randomized double-blind trials are required to evaluate other combinations of chemotherapy regimens, most importantly “pulsed” dactinomycin with the widely used 8-day methotrexate-folinic acid.[5] Finally, 8-day methotrexate-folinic acid is effective in treatment of low risk gestational trophoblastic neoplasia. A cycle of Methotrexate is estimated between $50 and $60. Patients and their families cannot always gather this amount. We rarely prescribe folinic acid in “rescue”; a bottle of folinic acid costing more expensive than a cycle of Methotrexate. Fortunately, we rarely observe side effects of methotrexate.
In this study, finally 94.3% of low-risk patients achieved remission after second line chemotherapy. In developing world cost of treatment remains a major concern. MTX is a relatively safe, effective and inexpensive drug and thereby more easily available to Senegalese patients. Second-line regimens are very difficult to implement in our context. Act D used in MAC and EMACO protocols is often obtained through donations. This drug whose effectiveness is recognized is not always available in our country. Moreover, its high price limits its access.
4.2. Treatment and outcome of high-risk gestational trophoblastic neoplasia (HRGTN)
Patients with high-risk GTN (FIGO stages II-III with score >7 and stage IV) should be treated with multiagent chemotherapy with or without adjuvant surgery and radiotherapy.[2,6,7]
The multiagent therapy of choice has changed over the years. In the 1970s and 1980s, MTX, ActD and cyclophosphamide or chlorambucil (MAC) were the first line treatment, and cure rates reached 63 to 71%. In the early 1980s, studies found that the regimen with cyclophosphamide, hydroxyurea, ActD, MTX/FA, vincristine and doxorubicin (CHAMOCA) increased primary remission to 82%. However, CHAMOCA had lower sustained primary remission rates, as well as greater toxicity, than the MAC regimen.[6,7]
In 1980, etoposide was found to be a very effective agent in the treatment of GTN. Regimens using this drug in combination with high doses of MTX, FA, Act D, cyclophosphamide and vincristine (EMA-CO) resulted in higher remission and survival rates.[6] The EMACO regimen became the first choice for the treatment of high-risk GTN because of its low toxicity and high complete response and survival rates.[7-9]
A cycle of EMACO regimen (d1, d2 and d8) is financially valued at $355. This represents more than the average wage of a Senegalese worker. In fact, the income of couples was very low in 68.5 % of cases. This situation leads to lack of normal monitoring of hCG which delays the diagnosis of gestational trophoblastic neoplasia and worsens the prognosis of these patients.
The particularity of our health system is the lack of health insurance.
Although some tests are supported, chemotherapy drugs are in charge of patients and their families. The problem is much more deeper and the solution is not at an individual level. It requires a profound reorganization of our health system. We cannot hope for cancer convincing results if drugs are in charge of patients. This inequity in care is our daily both in the treatment of gestational trophoblastic neoplasia than in other cancers such as breast cancer. We have developed several social strategies. Indeed, a social worker in our department regularly approaches some persons who accept willingly to sponsor some patients. But, the treatment is long and expensive; this approach has also shown the limits of its efficiency. Patients with short survival had already gestational trophoblastic neoplasia when admitted in our department, several months after uterine evacuation. HCG monitoring was not properly-insured. At admission, they had visceral metastases. Unable to achieve the chemotherapy drugs appropriate to the risk, we prescribed chemotherapy adapted to their means or no chemotherapy at all. Sometimes their general condition did not allow chemotherapy until death occurs.
5. Conclusion and Global Health Implications
Early adequate treatment ensures an excellent prognosis for patients with GTN. In low-income countries, difficulties encountered in diagnosis and treatments worsen the prognosis of GTN patients. GTN are highly chemo-curable diseases. The diagnosis of the disease at an early stage ensures good results and allows the country to avoid huge expenses at late stage. Early diagnosis and treatment is very critical given that Senegal is a low-income country and drugs used at late stage are not always available in our country. However, clinical trials are needed to find out affordable schedules or drugs for a better treatment.
Ethical Consideration:: This study was approved by the Dakar Teaching Hospital and the National Reference Centre of Gestational Trophoblastic Diseases.
Conflict of Interest: The authors declare no conflict of interests.
References
- FIGO staging for gestational trophoblastic neoplasia. International Journal of Gynaecology and Obstetrics. 2002;77:285-7.
- [Google Scholar]
- Current management of gestational trophoblastic diseases. Gynecological Oncology. 2009;112(3):654-62.
- [Google Scholar]
- Prévention du choricarcinome post-môlaire en milieu africain: exemple du Sénégal. La Lette du Gynécologue. 2005;307:8-14.
- [Google Scholar]
- Diagnosis, classification and treatment of gestational trophoblastic neoplasia. Revista Brasileira de Ginecologia e Obstetrícia. 2015;37(1):42-51.
- [Google Scholar]
- First line chemotherapy in low risk gestational trophoblastic neoplasia (Review) The Cochrane Collaboration. 2009;1:1-11.
- [Google Scholar]
- Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. American Journal of Obstetrics and Gynecology. 2011;204(1):11-8.
- [Google Scholar]
- Current management of gestational trophoblastic neoplasia. Hematology/Oncology Clinics of North America. 2012;26(1):111-31.
- [Google Scholar]
- Gestational trophoblastic disease: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annuals of Oncology. 2013;24(Suppl 6):vi39-50.
- [Google Scholar]
- Gestational trophoblastic neoplasia. Obstetrics and Gynecology Clinics of North America. 2012;39(2):195-212.
- [Google Scholar]





