Translate this page into:
Impact of Maternal Viral Suppression on Growth Patterns for HIV-Exposed Uninfected Infants in Kenya

*Corresponding author: Megan S. McHenry, MD, MS, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, Indiana, United States. Tel: 317-274-4143 msuhl@iu.edu
-
Received: ,
Accepted: ,
How to cite this article: Magerko K, Humphrey J, Songok J, Musick B, Alera JM, Kipchumba B, et al. Impact of maternal viral suppression on growth patterns for HIV-exposed uninfected infants in Kenya. Int J Matern Child Health AIDS. 2024;13:e005. doi: 10.25259/IJMA_656
Abstract
Background and Objective
Children born to mothers living with human immunodeficiency virus (HIV) are at risk for poor health outcomes but data characterizing these associations are limited. Our objective was to determine the impact of maternal viral suppression on growth patterns and malnutrition for infants who are HIV-exposed but uninfected (HEU).
Methods
We conducted a retrospective cohort analysis of clinical data for infants who were HEU and their mothers (September 2015 – March 2019) in Kenya. Infants were stratified based on maternal viral suppression status (≥ or <1000 copies/mL); t-tests were used to compare groups. Growth indicators were evaluated with Chi-square, Fisher’s exact, and area under the curve. Moderate-to-severe underweight status, stunting, and wasting were defined by weight-for-age (WFA), height-for-age (HFA), and weight-for-height (WFH), z-scores ≤2, and were used to define malnutrition. Multivariate logistic regression analyses were performed to evaluate potential associations with malnutrition indicators between WFH and HFA.
Results
Among 674 infants who were HEU, 48.7% were male and 85.0% had mothers who were virally suppressed. The median age at first and last clinic visits was 1.5 and 16.4 months, respectively. WFA and HFA z-scores over time differed by sex, and WFA and HFA differed based on maternal viral suppression (P < 0.05). Male infants had higher adjusted odds for stunted status, and as children aged, they had slightly increased odds of becoming underweight or stunted. Maternal viral suppression and timing of maternal antiretroviral therapy initiation in relation to the prevention of vertical transmission (PVT) enrollment did not significantly affect malnutrition indicators.
Conclusion and Global Health Implications
Maternal viral suppression status was not associated with increased odds of more severe malnutrition indicators in children who were HEU. However, overall growth patterns over time, measured by z-scores of growth indicators, did differ based on maternal viral suppression status, and to a lesser degree, by gender.
Keywords
Viral Suppression
Low- and Middle-Income Countries
Key and Vulnerable Populations
Viral Load Monitoring
Kenya
INTRODUCTION
Background of the Study
With the success of prevention of vertical transmission (PVT) programs, the global population of children who are human immunodeficiency virus (HIV)-exposed but uninfected (HEU) has grown to nearly 16 million.[1,2] While infants who are HEU do not acquire HIV, they are at risk for worse health outcomes compared to their unexposed peers.[3,4] Studies have shown higher rates of comorbid conditions and mortality for children who are HEU compared to unexposed peers.[3,5] A myriad of risk factors may contribute to these differences, including perinatal HIV exposure, exposure to antiretroviral therapy (ART), the challenges of being a member of an HIV-affected household, or a combination of these factors.[3] One such risk factor, higher maternal viral loads, has been found to increase the risk for worse morbidity and mortality in children who are HEU.[6]
Malnutrition is more commonly found in children who are HEU compared to those who are unexposed to HIV.[7–10] Optimal nutrition in early childhood creates the critical foundation necessary to minimize mortality and morbidity over the lifespan.[11,12] Globally, the deaths of children under five years of age are linked to undernutrition among 45% of the population,[12] and children with malnutrition have worse cognitive development, independent of other potentially confounding factors.[13] While the data are clear that children who are HEU have worse nutritional outcomes compared to their unexposed peers, the impact of maternal viral suppression on growth patterns for children who are HEU is not well understood.[9,14]
Objectives and Hypothesis of the Study
In this study, we examined the impact of perinatal maternal viral suppression on the growth patterns of infants who are HEU and, secondarily, determined the rates of malnutrition in this population. We hypothesized that infants who are HEU and born to mothers who are not virally suppressed would have worse growth patterns and malnutrition rates compared to those born to mothers who are virally suppressed.
METHODS
Study Design
We conducted a retrospective cohort study using electronic medical record (EMR) data from infants who were HEU and their mothers. This study was performed as a secondary analysis of data originally obtained for pregnant and postpartum women living with HIV,[15] who were identified at the time of their first clinic visit, or previously, as having a positive test for HIV. The Institutional Research and Ethics Committee (IREC) approval at Moi University in Kenya was obtained. The study received a waiver for patient-level consent from the Moi IREC and was exempted from Indiana University Institutional Review Board because de-identified, routinely collected data were utilized for the analysis.
Study Setting
Infants and mothers jointly received integrated HIV care through the PVT services and associated maternal-child health clinics at Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya, from September 2015 to March 2019. For infants, this preventative treatment included HIV prophylaxis, growth monitoring, vaccinations, infectious disease prevention/treatment, and nutritional advice.[16] Further, information about typical infant visits at MTRH during a similar time frame is detailed in Deathe et al., 2022.[17] The HIV care and research infrastructure are a product of the Academic Model Providing Access to Healthcare (AMPATH) program, an over 30-year partnership between Moi University School of Medicine, MTRH, and a consortium of North American academic medical centers led by Indiana University School of Medicine.[18] AMPATH contributes data to the East Africa international epidemiology databases to Evaluate AIDS consortium.[19] All AMPATH facilities, including MTRH, provide standard-of-care HIV treatment services based on national guidelines.[20]
Standards of Care at Time of Study
As per the World Health Organization (WHO) and Kenyan HIV treatment guidelines at the time of our study, first-line ART was efavirenz-based (typically tenofovir disoproxil fumarate + lamivudine + efavirenz) for women 15 years and older of childbearing age, including during pregnancy.[21-23] Second-line ART was based on protease inhibitors (PIs) (atazanavir or lopinavir) plus ritonavir regimen. National guidelines recommended obtaining a viral load at the first antenatal visit (if the woman was already on ART), and then every six months thereafter (or every six months after ART initiation) while pregnant or breastfeeding, as long as the viral load remained <1,000 copies/mL. If the viral load was ≥1,000 copies/mL, additional counseling and support were provided to ensure excellent ART adherence,[22] and the viral load was recommended to be repeated after three months. If the viral load remained ≥1,000 copies/mL at three months, a second-line regimen was initiated. HIV testing for infants was conducted within the first six weeks of life per the guidelines. If the first HIV DNA polymerase chain reaction testing was positive, the infant was to be presumed HIV-infected. If it was negative, the infant was considered HIV-exposed with repeat testing conducted at six months and 12 months of age; at 18 months of age, HIV antibody testing was completed according to the guidelines.[22] The recommended ART prophylaxis initiated at birth was six weeks of zidovudine and nevirapine, with the latter recommended to continue until six weeks after cessation of breastfeeding.[22]
Definitions
To determine malnutrition status, we converted child age, height, and weight into z-scores for weight-for-height (WFH), weight-for-age (WFA), and height-for-age (HFA), according to WHO’s reference means.[24] Moderate-to-severe underweight, stunting, and wasting status were defined as z-scores ≤ −2 standard deviations (SD) for WFA, HFA, and WFH using WHO calculations.[24,25]
Perinatal maternal viral suppression, henceforth referred to as maternal viral suppression, was defined as <1,000 copies/mL. If the mother had a viral load of ≥1,000 copies/mL at any point between 6 months before her first prenatal visit or 18 months after delivery, then she was considered not suppressed. Maternal ART adherence was determined by clinicians at each visit and categorized as good or poor.
Data Management
Clinical data from routine care visits were collected on paper-based forms and then entered by data clerks into the AMPATH EMR system. Recording errors were minimized by excluding infants with weight changes of >3 kg/month or height changes of >10 cm/month.[26] Infants with heights that were less than previous recordings by ≥2 cm were also excluded from the study. Z-scores for WFA, HFA, or WFH that changed ≥2 units/month or had absolute z-scores of >6 units were also excluded from the study. These criteria were determined based on WHO growth charts and clinical judgment.
Statistical Analysis
Study characteristics were calculated as means and SD or percentages. t-tests were utilized to compare suppressed and not suppressed mother and infant characteristics. Percentages for underweight status, stunting, and wasting at 6, 12, and 18 months of age (with a range of 2 months allowed for each time point) were calculated, and we utilized Chi-square and Fisher’s exact tests. When we compared the percentages for malnutrition at these three time points, there was no adjustment made when using Chi-squared and Fisher’s exact tests. Graphic representations of WFA, HFA, and WFH z-scores from the first clinic visit to the last visit available were generated. We completed area under the curve analysis for WFA, HFA, and WFH to compare male and female sexes, as well as not suppressed and suppressed maternal viral load status. We also performed multivariate logistic regression to determine the adjusted odds ratios for moderate-to-severe underweight status, stunting, and wasting based on perinatal maternal viral suppression. Since infants were enrolled at different ages and due to extensive missing data for prematurity, we controlled for infant WFA z-score at first clinic visit, sex, maternal age at time of delivery, infant age at each time point, and ART before PVT enrollment. All analyses were completed in SAS Institute Inc. 2013. Base SAS® 9.4 Procedures Guide. Cary, NC: SAS Institute Inc. and R v 4.04. Statistical significance was set to α < 0.05.[27]
RESULTS
Sociodemographic Characteristics
Infants were selected based on their mothers receiving integrated HIV care from an MTRH clinic between August 2015 and September 2017. Mothers were included in this cohort if they were living with HIV and had a measured viral load within six months before or after their delivery date. Infants were included if they were born at least 18 months (at the timing of confirmatory HIV testing) before database closure and had both height and weight measurements present in the EMR. Of the 845 potentially eligible women, 114 were not linked to an infant and were therefore excluded [Supplemental Figure 1]. This resulted in 735 infants linked to 731 mothers, including four sets of twins. As this study focuses on infants who were HIV-exposed but not infected, nine infants were excluded due to HIV infection. Data were not available on HIV status for 24 infants and perinatal viral suppression data were not available for 28 mothers. The final sample included 674 infants and 670 mothers.
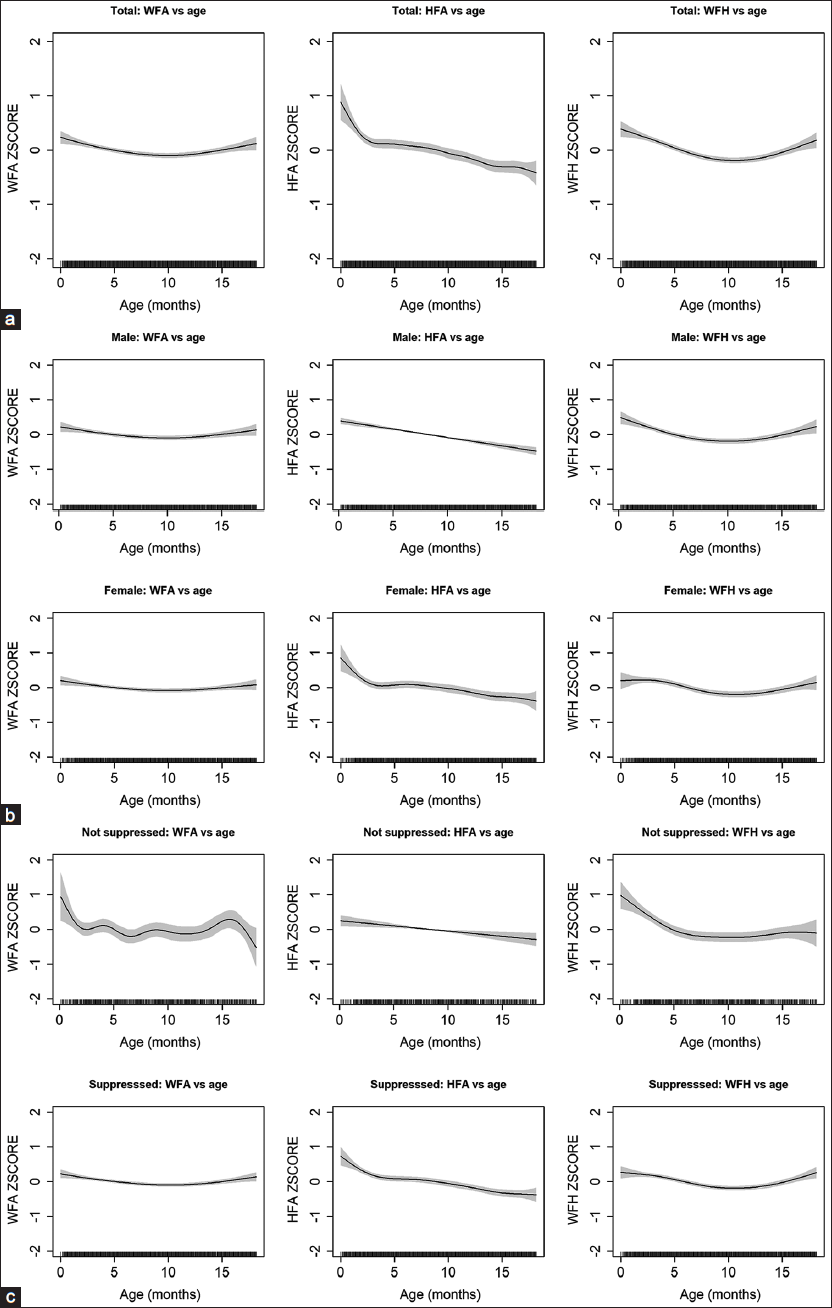
- Z-scores with age in years (a) for all HIV-exposed but uninfected infants, (b) by male and female sex, and (c) by maternal viral suppression. The shaded region represents the 95% confidence interval. WFA: Weight-for-age, HFA: Height-for-age, WFH: Weight-for-heightMagerko, et al.: HIV-Exposed Uninfected Growth Patterns in KenyaMagerko, et al.: HIV-Exposed Uninfected Growth Patterns in Kenya
There were 674 infants who were HEU included in the final sample, with approximately half male (49%) and with a median age of 1.5 months (interquartile range [IQR] 1.4, 1.8) at first clinic visit and 16.4 months (IQR 15.1, 17.3) at last clinic visit [Table 1]. The mothers had a median maternal age of 32.2 (IQR 28.1, 36.3) at delivery; 87% were on ART before prevention of mother-to-child transmission (PMTCT) enrollment; and 98% were considered to have good adherence to ART. Mothers who were virally suppressed made up 85% of the population of dyads studied.
| Infant characteristics (mean, SD) | Total | Non-suppressed | Suppressed | P-value |
|---|---|---|---|---|
| n = 673 | n = 101 | n = 572 | ||
| Age in months at first visit, mean (SD) median (IQR) (n = 673) | 1.7 (1.3) | 1.8 (1.2) | 1.7 (1.3) | 0.50 |
| 1.5 (1.4, 1.8) | 1.5 (1.4, 1.7) | 1.5 (1.4, 1.8) | ||
| Age in months at last visit, mean (SD) median (IQR) (n = 673) | 15.5 (3.1) | 15.4 (3.0) | 15.5 (3.1) | 0.59 |
| 16.4 (15.1, 17.3) | 16.2 (14.8, 17.3) | 16.4 (15.2, 17.4) | ||
| Male (%), (n = 673) | 328 (48.7) | 51 (50.5) | 277 (48.4) | 0.70 |
| WFA z-scorev1 (n = 667) | −0.7 (1.4) | −0.8 (1.5) | −0.7 (1.4) | 0.32 |
| HFA z-scorev1 (n = 561) | −0.6 (1.6) | −1.1 (1.8) | −0.6 (1.6) | 0.01 |
| WFH z-scorev1 (n = 557) | 0 (1.5) | 0.4 (1.5) | 0 (1.5) | 0.02 |
| No. of clinic visits§ (n = 673) | 9.6 (2.8) | 10.0 (3.1) | 9.5 (2.7) | 0.07 |
| Length of follow-up in months (n = 673) | 13.8 (3.3) | 13.6 (3.3) | 13.8 (3.3) | 0.68 |
| Mean no. of clinic visits per month (n = 673) | 0.7 (0.3) | 0.8 (0.2) | 0.7 (0.3) | 0.37 |
| Maternal characteristics (n, %) | n = 669 | n = 101 | n = 568 | |
| Maternal age at delivery in years mean (SD), median (IQR) (n = 669) | 32.1 (6.0) | 30.7 (6.8) | 32.3 (5.8) | 0.02 |
| 32.2 (28.1, 36.3) | 30.8 (27.1, 35.3) | 32.4 (28.3, 36.5) | ||
| Initiation of ART before PMTCT enrollment, yes (n = 669) | 581 (86.9) | 94 (93.1) | 487 (85.7) | 0.04 |
| Adherence on ART† (n = 666) | ||||
| Good | 652 (97.9) | 95 (96.0) | 557 (98.2) | 0.18 |
| Poor | 9 (1.4) | 3 (3.0) | 6 (1.1) | |
| Not on ART | 5 (0.8) | 1 (1.0) | 4 (0.7) | |
| ART regimen* (n = 667) | ||||
| NNRTI | 569 (85.3) | 69 (68.3) | 500 (88.3) | <0.01 |
| PI | 74 (11.1) | 23 (22.8) | 51 (9.0) | |
| Switch during pregnancy | 24 (3.6) | 9 (8.9) | 15 (2.7) | |
V1At first visit, §Within first 18 months of life, *During pregnancy, †Clinician reported at first prenatal visit. Bolded P-values indicate P < 0.05. ART: Antiretroviral therapy, PMTCT: Prevention of Mother-to-child transmission of HIV, NNRTI: Non-nucleoside reverse transcriptase inhibitors, PI: Protease inhibitor, SD: Standard deviation, IQR: Interquartile range, WFA: Weight-for-age, HFA: Height-for-age, WFH: Weight-for-height, n: the number of individual participants included within that group for analysis.
Main Variable Results
Significant differences were found between virally not suppressed and suppressed mothers for maternal ART regimen (P < 0.01). Mothers who were not suppressed were more likely to be on a PI-based regimen (i.e., lopinavir or atazanavir, 23% vs. 10%) and to have switched ART regimens during pregnancy (8% vs. 3%). Women who were not suppressed were also more likely to have initiated ART before PVT enrollment. Infant initial WFA, HFA, and WFH did not differ between not suppressed and suppressed maternal viral load groups, nor did the length of follow-up or mean number of clinic visits per month.
When comparing rates of moderate-to-severe malnutrition in infants at 6, 12, and 18 months of age-stratified by maternal viral suppression status, statistically significant differences were found at 6 months indicating underweight status (P < 0.05) for infants of non-suppressed mothers [Table 2]. The rates of underweight status at 12 months, stunting at 6 months, and wasting at 6 months suggested a trend toward higher rates of malnutrition among infants born to mothers who were not virally suppressed (P < 0.20).
| Age | Not suppressed (n, %) | Suppressed (n, %) | P-value |
|---|---|---|---|
| Percentage underweight | |||
| 6 months (n = 592) | 20 (22.7) | 64 (12.7) | 0.01* |
| 12 months (n = 537) | 21 (25.3) | 85 (18.7) | 0.17 |
| 18 months (n = 380) | 8 (15.1) | 44 (13.5) | 0.75 |
| Percentage stunted | |||
| 6 months (n = 570) | 23 (27.1) | 78 (16.1) | 0.11 |
| 12 months (n = 519) | 20 (25.6) | 105 (23.8) | 0.73 |
| 18 months (n = 367) | 16 (31.4) | 92 (29.1) | 0.74 |
| Percentage wasted | |||
| 6 months (n = 563) | 10 (11.9) | 36 (7.5) | 0.18 |
| 12 months (n = 519) | 9 (11.7) | 37 (8.4) | 0.34 |
| 18 months (n = 364) | 5 (9.8) | 22 (7.0) | 0.48 |
Z-score plots are seen in Figure 1a for all infants who are HEU for malnutrition indicators including WFA, HFA, and WFH. The plots were further stratified by male and female sex [Figure 1b], and the test statistics when comparing the two groups were as follows: WFA was 0.03 (P = 0.01), HFA was 0.06 (P < 0.01), and WFH was 0.01 (P = 0.24). In Figure 1c, z-scores were examined by maternal viral suppression with significant differences seen in the area under the curve for WFA (test statistic = 0.07, P = 0.04), HFA (test statistic = 0.11, P = 0.01), and WFH (test statistic 0.06, P = 0.02).
Covariates Results
Infants had decreased odds of being stunted, underweight, and wasted if they had higher WFA z-scores at their first clinic visit (every unit increase in z-score was associated with 30%, 43%, and 61% lower odds of underweight, stunted status, and wasted status, respectively). Infants were also more likely to be underweight or stunted with each additional month of age. They had increased odds of being stunted if they were male [Table 3]. Maternal viral suppression and ART initiation Antenatal HIV Care enrollment were not associated with increased odds of moderate-severe malnutrition.
| Adjusted odds ratio (95% CI) | |||
|---|---|---|---|
| Underweight (WFA z-score ≤ 2) | Stunted (HFA z-score ≤ 2) | Wasted (WFH z-score ≤ 2) | |
| Maternal age (years) | 1.02 (0.99, 1.05) | 1.01 (0.99, 1.04) | 1.01 (0.99, 1.04) |
| WFA z-scorev1 | 0.30 (0.26, 0.34)* | 0.43 (0.38, 0.48)* | 0.61 (0.55, 0.69)* |
| Male | 1.05 (0.72, 1.53) | 1.39 (1.01, 1.90)* | 1.22 (0.89, 1.68) |
| Infant age at each time point (months) | 1.02 (1.00, 1.04)* | 1.06 (1.04, 1.08)* | 1.00 (0.98, 1.02) |
| ART initiation before PMTCT enrollment | 1.02 (0.56, 1.85) | 1.22 (0.74, 2.00) | 0.95 (0.58, 1.56) |
| Suppressed maternal viral load | 0.61 (0.37, 1.02) | 0.71 (0.46, 1.08) | 0.69 (0.45, 1.05) |
DISCUSSION
While at all-time points (6, 12, and 18 months), there were higher proportions of children exposed to maternal viral non-suppression with moderate-severe malnutrition (underweight status, stunting, and wasting) compared to those with maternal viral suppression, only underweight status at six months of life met statistical significance. Male sex, WFA z-score at first infant clinic visit, and infant’s age were also associated with malnutrition.
Few studies examining growth in children who are HEU examine maternal viral suppression, and existing data on this topic have been mixed. A study in Zimbabwe found that 28.5% of infants who were HEU were malnourished by six weeks of age, with increased odds of stunting and lower birth weight when compared to HIV unexposed infants, but no association was found between malnutrition and maternal viral loads.[5] In South Africa, higher maternal viral loads were associated with lower weight velocity z-scores,[14] while another study found maternal viral suppression associated with increased rates of stunting.[9] Our study contributes important findings to better understand the role of maternal viral suppression on infant growth outcomes.
Within our study, we did not find any differences in nutritional status based on whether ART had been initiated before pregnancy or not. In Rwanda, the duration of maternal ART was associated with increased rates of stunting.[9] In Nairobi, Kenya, growth differences for infants who are HEU showed decreased WFA and HFA z-scores with longer exposure to maternal ART.[26] While ART is critical for PVT and the control of HIV throughout the life course, pharmacovigilance and further research are needed to understand the impact of maternal ART on infant outcomes.
Male infants in our study who were HEU had higher odds of being underweight, stunted, and wasted, which is consistent with our previous data from Kenya and the above-mentioned study in Zimbabwe.[5,8] Another study in Kenya demonstrated a greater disadvantage in food intake and malnutrition amongst girls;[28,29] however, stunting has also been shown to be more common for male infants with HIV in Botswana and across Sub-Saharan Africa.[30,31] A recent meta-analysis found that males experience undernutrition (wasting, stunting, and underweight) more commonly across age groups in most regions of the world, including Africa.[28] These differences may be due to increased risk for males in countries with overall lower socioeconomic status,[31] higher vulnerability for morbidity and mortality for male infants, differences in parenting and resource allocation, and sex hormone differences.[28] Future research should continue to evaluate sex differences in the population of infants who are HEU to understand why males may be more at risk for malnutrition and whether different interventions may be needed for this population.
Strengths and Limitations of the Study
With this study’s observational nature, there are some limitations to note. Prematurity was not well-documented within the data set. WFA z-score at the initial visit, was utilized to account for possible differences due to prematurity, but a true measure of prematurity would have been preferred. In addition, our large sample had relatively few women who were virally non-suppressed (<15%), despite the broad criteria (viral load ≥1,000 copies/mL at any point between 6 months before pregnancy and 18 months post-partum), making it difficult to find meaningful patterns and associations within the data. However, this is a welcomed challenge and a testament to the success of their clinical care. Other important factors such as the specific infant antiretroviral prophylaxis, feeding type, number of siblings, spacing, geographic location, and socioeconomic status were unable to be assessed given the EMR data collection method. However, this large data set yielded important results to guide us in understanding the growth trajectories of children who are HEU.
CONCLUSION AND GLOBAL HEALTH IMPLICATIONS
Maternal viral suppression was associated with slightly improved growth outcomes in children who were HEU. With our study, we also found that the male sex, lower WFA z-score at the first clinic visit, and increasing age are all associated with increased odds of malnutrition. Identifying areas for potential intervention is key to improving the clinical outcomes of children who are HEU. Given the growing population of infants that are HEU, born each year, assessing areas for intervention to improve growth and development in this population is pivotal to our local and global society. Optimizing maternal viral suppression and providing support for male infants and children as they age may be important considerations for PVT. Growth monitoring should be a part of all pediatric HIV care programs, and more research is needed to understand factors associated with poor nutritional outcomes, to promote optimal health outcomes among infants who are HEU.
Key Messages
-
Infants born to non-virally suppressed mothers living with HIV are at increased risk for being underweight at six months.
-
Male infants were more likely to be wasted or stunted across both groups.
-
With each passing month, increase in age, infants had a higher odds of malnutrition.
Acknowledgments
We would like to thank Mr. Justin Kipsang and the clinical staff at Moi Teaching and Referral Hospital for collecting the data that made this analysis possible. We would also like to thank Melissa Thomas for her careful review and editing of the manuscript.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest
The authors declare no competing interests.
Financial Disclosure
Nothing to declare.
Funding/Support
The International Epidemiology Databases to Evaluate AIDS (IeDEA) is supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, the Fogarty International Center, and the National Library of Medicine: East Africa, U01AI069911. A National Institutes of Mental Health K23 Mentored Career Development Award (K23MH116808, PI: McHenry) provided salary support for the senior author during the analysis of this study.
Ethics Approval
Institutional Research and Ethics Committee approval at Moi University in Kenya and an exemption from Institutional Review Board review at Indiana University were obtained.
Declaration of Patient Consent
The patient’s consent was not required because the patient’s identity was not disclosed or compromised.
Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
This work is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
SUPPLEMENTAL FIGURE
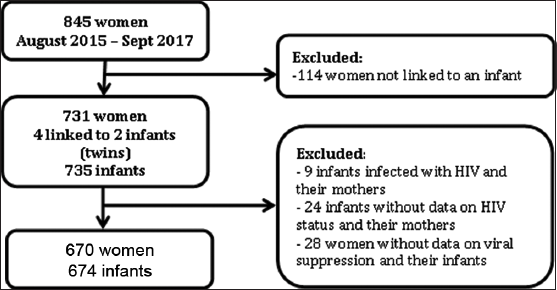
- Sample Selection Process. HIV: human immunodeficiency virus.
REFERENCES
- Estimates of the global population of children who are HIV-exposed and uninfected, 2000-18: A modelling study. Lancet Glob Health. 2020;8(1):e67-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- UNAIDS SPECTRUM 2022 Estimates. 2022.
- HIV-exposed uninfected children: A growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176(1):11-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of maternal HIV infection on infant development and outcomes. Front Virol. 2022;2:885246.
- [Google Scholar]
- Growth trajectories of HIV exposed and HIV unexposed infants. A prospective study in Gweru, Zimbabwe. Glob Pediatr Health. 2021;8:2333794X21990338.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41(11):1654-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Growth of HIV-exposed uninfected, compared with HIV-unexposed, Zambian children: A longitudinal analysis from infancy to school age. BMC Pediatr. 2017;17(1):80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Growth of young HIV-infected and HIV-exposed children in Western Kenya: A retrospective chart review. PLoS One. 2019;14:e0224295.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Longitudinal interrelationship between HIV viral suppression, maternal weight change, breastfeeding, and length in HIV-exposed and uninfected infants participating in the Kabeho Study in Kigali, Rwanda. Ann Epidemiol. 2021;53:1-6.e1.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HIV-Exposed, Uninfected infants in Uganda experience poorer growth and body composition trajectories than HIV-unexposed infants. J Acquir Immune Defic Syndr. 2020;85(2):138-47.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Organ. 2000;78(10):1207-21.
- [PubMed] [PubMed Central] [Google Scholar]
- 2023. Malnutrition. WHO: Geneva; [Accessed 2023 Jan 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/malnutrition
- Malnutrition at age 3 years and lower cognitive ability at age 11 years: Independence from psychosocial adversity. Arch Pediatr Adolesc Med. 2003;157(6):593-600.
- [CrossRef] [PubMed] [Google Scholar]
- HIV infection, viral load, low birth weight, and nevirapine are independent influences on growth velocity in HIV-exposed south african infants. J Nutr. 2014;144(1):42-8.
- [CrossRef] [PubMed] [Google Scholar]
- Retention in care and viral suppression in the PMTCT continuum at a large referral facility in Western Kenya. AIDS Behav. 2022;26(11):3494-505.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mother and child health handbook. Kenya: Republic of Kenya Ministry of Health; 2020. [Accessed 2023 Nov 1]. Available from: https://familyhealth.go.ke/wp-content/uploads/2020/11/Mother-Child-Health-Handbook-MOH-September-2020.pdf
- Preventive health service coverage among infants and children at six maternal-child health clinics in Western Kenya: A cross-sectional assessment. Matern Child Health J. 2022;26(3):522-9.
- [CrossRef] [PubMed] [Google Scholar]
- Partnerships in international health. The Indiana University-Moi University experience. Infect Dis Clin North Am. 1995;9(2):453-5.
- [PubMed] [Google Scholar]
- Cohort profile: The International epidemiological Databases to Evaluate AIDS (IeDEA) in sub-saharan Africa. Int J Epidemiol. 2012;41(5):1256-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Guidelines on use of antiretroviral drugs for treating and preventing HIV infection: Rapid advice. [Accessed 2023 Nov 1]. Available from: http://guidelines.health.go.ke
- Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya. Nairobi, Kenya: NASCOP; 2016.
- Guidelines on use of antiretroviral drugs for treating and preventing HIV infections in Kenya. 2018.
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach (2nd ed). Geneva: World Health Organization; 2016.
- WHO global database on child growth and malnutrition. [Accessed 2023 Nov 1]. Available from: https://apps.who.int/nutgrowthdb/en
- standard deviation of anthropometric Z-scores as a data quality assessment tool using the 2006 WHO growth standards: A cross country analysis. Bull World Health Organ. 2007;85(6):441-8.
- [CrossRef] [PubMed] [Google Scholar]
- Growth patterns and their contributing factors among HIV-exposed uninfected infants. Matern Child Nutr. 2021;17(2):e13110.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 2021. R: A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing; [Accessed 2023 Nov 1]. Available from: https://www.R-project.org/
- Boys are more likely to be undernourished than girls: A systematic review and meta-analysis of sex differences in undernutrition. BMJ Glob Health. 2020;5(12):e004030.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gender inequality in food intake and nutritional status of children under 5 years old in rural Eastern Kenya. Eur J Clin Nutr. 2011;65(1):26-31.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence and determinants of short stature in HIV-infected children. J Int Assoc Provid AIDS Care. 2014;13(6):529-33.
- [CrossRef] [PubMed] [Google Scholar]
- Boys are more stunted than girls in Sub-Saharan Africa: A meta-analysis of 16 demographic and health surveys. BMC Pediatr. 2007;7:17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





