Translate this page into:
Integration of Neonatal and Child Health Interventions with Pediatric HIV Interventions in Global Health
*Corresponding author email: Briasmith@usaid.gov
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background/Objectives:
In the last decade, many strategies have called for integration of HIV and child survival platforms to reduce missed opportunities and improve child health outcomes. Countries with generalized HIV epidemics have been encouraged to optimize each clinical encounter to bend the HIV epidemic curve. This systematic review looks at integrated child health services and summarizes evidence on their health outcomes, service uptake, acceptability, and identified enablers and barriers.
Methods:
Databases were systematically searched for peer-reviewed studies. Interventions of interest were HIV services integrated with: neonatal/child services for children <5 years, hospital care of children <5 years, immunizations, and nutrition services. Outcomes of interest were: health outcomes of children <5 years, integrated services uptake, acceptability, and enablers and barriers. PROSPERO ID: CRD42017082444.
Results:
Twenty-eight articles were reviewed: 25 (89%) evaluated the integration of HIV services into child health platforms, while three articles (11%) investigated the integration of child health services into HIV platforms. Studies measured health outcomes of children (n=9); service uptake (n=18); acceptability of integrated services (n=8), and enablers and barriers to service integration (n=14). Service integration had positive effects on child health outcomes, HIV testing, and postnatal service uptake. Integrated services were generally acceptable, although confidentiality and stigma were concerns.
Conclusion and Global Health Implications:
Each clinical “touch point” with infants and children is an opportunity to provide comprehensive health services. In the current era of flat funding levels, integration of HIV and child health services is an effective, acceptable way to achieve positive child health outcomes.
Keywords
Africa
HIV
PITC
Child health services
PMTCT
neonatal health
Literature review
Immunization program
1. Background
Sub-Saharan Africa remains the region with the highest under-five mortality rate in the world. In 2016, the region had an average under-five mortality rate of 79 deaths per 1,000 live births.1 This translates to 1 child in 13 dying before his or her fifth birthday – 15 times higher than the average ratio of 1 in 189 in high-income countries. Among newborns, approximately 1 child in 36 dies in the first month of life, compared to a ratio of 1 in 333 in high-income countries.2 HIV directly affects child morbidity and mortality, especially in countries that have a high HIV prevalence,3 and the risk for each is higher among children before antiretroviral therapy (ART) initiation and even greater in those children who are immunosuppressed.4
While gains in the pediatric HIV response have been made due to the scale up of prevention of mother-to-child-transmission (PMTCT) programs, pediatric case identification, and provision of pediatric ART, the response for children still lags compared with that of adults. For example, despite an estimated 210,000 HIV infections averted in 2017 due to successful PMTCT programs, there were still approximately 180,000 infants who were vertically infected with the virus in the same year.5 Finding children living with HIV (CLHIV) remains challenging, and children are also less likely to receive ART than adults (52% vs 59%).5 Rapidly identifying the HIV status of children and linking them to care and treatment is crucial for child survival, especially among HIV-exposed infants (HEI) where peak mortality from HIV infection occurs between six weeks to four months of age.6-8 Despite the potential for case finding during provision of services at child survival entry points, vertical programming has historically missed these opportunities, resulting in poor survival outcomes for children with comorbidities.3
In the last decade, many regional and global strategies, plans, and guidelines have called for the integration of HIV and maternal, neonatal and child survival platforms to reduce missed opportunities and to improve child health.9-14 Integration is defined by the World Health Organization as the “management and delivery of health services, where clients receive a continuum of preventive and curative services according to their needs over time, and across different levels of the health system”.15 Often, integration is considered as a comprehensive approach, such as a “one-stop-shop”.16 The international community committed to UNICEF’s Double Dividend initiative17 to accelerate achievement of the dual goals of ending pediatric HIV and AIDS and improving child survival through targeted joint interventions.17 Through these calls to action, countries with generalized HIV epidemics have been encouraged to identify efficiencies in service delivery platforms and to optimize each clinical encounter to bend the HIV epidemic curve.
There is some evidence that integration has been effective in improving outcomes for children. Countries who fully implemented the Integrated Management of Childhood Illness (IMCI) strategy demonstrated a nearly four-fold higher rate of achieving the Millennium Development Goal to reduce under-five mortality by two thirds.18 Full implementation of IMCI is defined by all three components: (1) improving health worker skills, (2) strengthening health systems and (3) improving family and community practices. This work highlighted that investments in health systems strengthening are critical to the successful implementation of integrated child health and HIV services.
The present era of the Sustainable Development Goals (SDG) provides an opportunity to focus on integrated programming to meet global commitments to end preventable newborn and child mortality. At the national level, studies from family planning and HIV services have shown that integration has the potential to optimize limited funding within health systems.19 At the facility level, numerous provider-child interactions can be synergized to allow integration of child health and HIV and reduce missed clinical opportunities, as shown in Figure 1. Despite these facilitators, integration has not been uniformly implemented across facilities, countries, or regions, hampered by the lack of effective examples of models of integration.
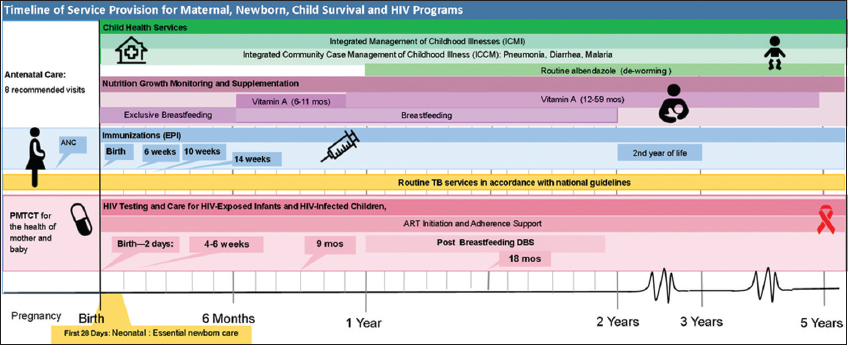
- Timeline of Service Provision for Maternal, Newborn, Child Survival and HIV Programs. Notes: In sub-Saharan Africa, progress on reducing preventable child deaths and increasing antiretroviral therapy (ART) coverage in children living with HIV (CLHIV), while gaining ground, has been slower than desired. Too often, the delivery of health programs for children are “siloed” in specific intervention areas focused on HIV, TB, Nutrition, or Immunizations. This is often a product of how Ministries of Health or clinics are organized, or how international donor funding streams are allocated and regulated. “Siloed” or non-integrated programs can lead to mother-child dyad being reached with one type of intervention, while being missed for another. Programmers and implementers could better optimize each clinical “touch-point” with a mother-child dyad to provide more integrated and comprehensive services. Abbreviations and acronyms: ART-Antiretroviral Therapy, DBS-Dried Blood Spot, EPI-Expanded Program on Immunization, HIV- Human Immunodeficiency Virus, PMTCT-Prevention of Mother to Child Transmission, TB-Tuberculosis.
This systematic review strengthens the evidence base for integration by summarizing study findings of integrated HIV services with neonatal and child services, specifically assessing the effects on child health outcomes and service uptake, acceptability by patient and provider, and enablers and barriers to service integration. The findings from this systematic review can be used as a resource by policymakers and programmers to understand the effectiveness, the efficiency, and the results on service uptake afforded by integration of child healthcare services.
2. Methods
2.1. Search strategy
The protocol for this review was published on PROSPERO (CRD42017082444). A systematic search of peer-reviewed published articles was conducted in PubMed and EBSCO Academic Search Complete as of 9/19/2016. The search syntax used is shown in Table 1.
| (((((pediatric OR paediatric OR child* OR infant* OR neonat* OR newborn OR under-five OR U-5)) AND (HIV AND (testing OR diagnosis OR treatment OR ART))) AND (postnatal OR PNC OR postpartum OR immuniz* OR immunis* OR EPI OR RED OR nutrition* OR growth monitor* OR supplemental feed* OR malnutrition OR IYCF OR CMAM OR CTC OR developmental assessment OR IMCI OR iCCM OR ((pediatric OR paediatric OR child* OR infant* OR neonat* OR newborn OR under-five OR U-5) AND (community-based OR primary care OR center OR facility OR clinic OR ward OR hospital))))) |
2.2. Inclusion and exclusion criteria
Figure 2 depicts the literature search methodology of the review. One reviewer completed an initial title review to identify potentially relevant publications and to remove duplicates. Building off of the key interventions highlighted in the Double Dividend, reviewers focused the search on four priority interventions listed in Table 2. Two reviewers conducted an abstract review of the remaining studies and achieved consensus to exclude abstracts that did not meet the inclusion and exclusion criteria (Table 2). The same process was executed for the full text review. Data were extracted from each article for analysis, including: Author (1st), Year, Country, Geographic Setting (example. Urban, rural), Location of Services (Community or Facility), Study Design, Sample Size, Participants (Children, Infants, Mothers, Mother-infant pairs), Primary Outcome(s) of Interest, Results for primary outcome(s) and significance, if applicable (e.g. CI, p-value), HIV Intervention (Diagnosis, treatment, retention, PMTCT), Child Health Intervention, (HIV integrated X package of services, i.e. Nutrition, EPI, etc), HIV Testing Type (EID DNA PCR, Pediatric, etc.) (if relevant) DNA PCR, Ab, or Both, Treatment Eligibility & Regimen (if relevant), Context (Direction of integration: HIV service into child health program or child health intervention into HIV program), Effect on Services, Demand Side Barriers, Demand Side Enablers, Health System Barriers, Health System Enablers, Secondary Outcomes, Other Notes, Funding Source and Agency. After initial data extraction, two reviewers examined each column to ensure consistency of approach in data extraction across all articles. Extracted data was then reviewed in relation to the four priority interventions and columns were analyzed to identify results.
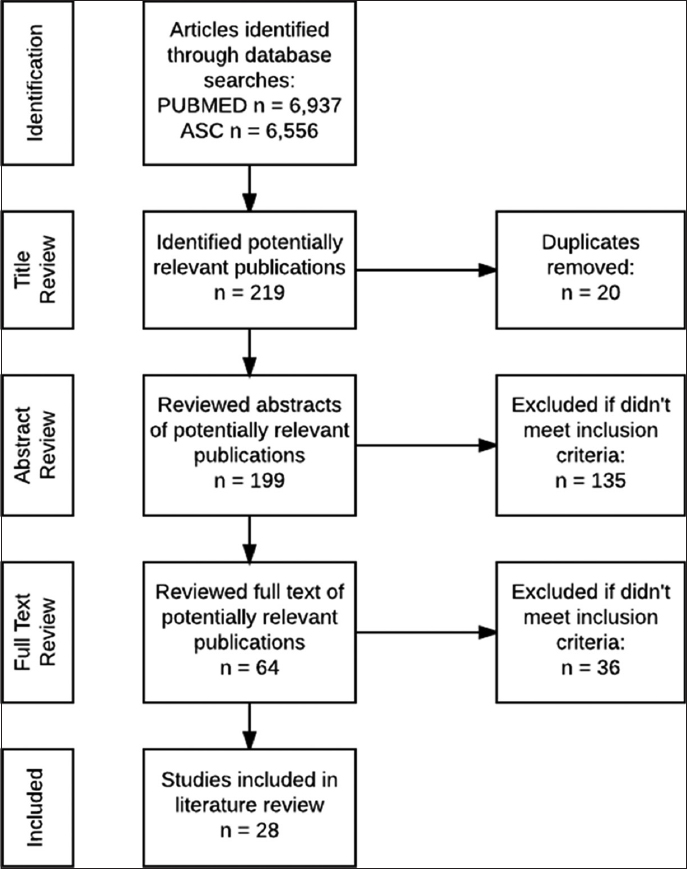
- Study Flowchart
| Inclusion criteria | Exclusion criteria |
|---|---|
| Published between 1/1/20089/19/2016 | No intervention |
| Study conducted in Africa | Non English literatures |
| Study focused on children<5 years old | Not focused on children |
| Containing at least 1 of 4 priority interventions: a) postnatal follow up that includes integrated HIV, neonatal and child survival interventions; b) expanded program for immunizations (EPI) services that incorporate HIV testing services; c) nutrition center services integrated with HIV testing and growth monitoring of HIV-exposed children; and d) pediatric inpatient and ambulatory care services that include provider initiated HIV testing for children being treated at these locations. | |
2.3. Quality assessment
All studies were assessed for quality using the National Institutes of Health Study Quality Assessment Tools (NHLBI, 2014). Assessments were stratified by study design, which included observational cohort and cross-sectional, controlled intervention and pre-post studies (Table 3). Out of the 28 studies included in this review, 13 were good quality, 11 were fair quality and four studies were poor quality. No studies were eliminated on the basis of quality.
3. Results
3.1. Overview of studies included
The full text review yielded 28 articles that met the inclusion criteria, including at least one of the four priority interventions as captured in Table 4. Study characteristics are shown in Table 5, and the results of each article in Tables 6-9.
The majority of interventions documented in these articles were conducted in East and Southern Africa, with only three articles documenting interventions in West or Central Africa. The articles described a range of study design methodologies, including cohort prospective (n=13), cohort retrospective (n=6), controlled (n=5), pre-post with no control (n=3), and one qualitative assessment (Table 5).
The articles represented a total of 84,018 study participants, including 19,429 mothers, and 64,589 infants and children. All studies included infants or children as participants, and 14 studies included linked mothers as participants. For this reason, we have reported maternal outcomes when relevant for child outcomes. Over half of the studies described were implemented in urban settings (n=15), with the remainder implemented in peri-urban (n=5), rural (n=4) or a combination of settings (n=4). Five of the 28 articles reviewed described community-based interventions, and the remainder described facility-based interventions (Table 5).
The included articles described integration of several types of HIV services into a variety of child health interventions. HIV services included: diagnostic testing for the mother-child dyad; linkage to ART; and PMTCT services, including services for HEI. Child health interventions included: nutrition interventions; inpatient services; and outpatient services such as immunizations, and tuberculosis (TB) care.
The 28 included articles assessed integration of services for infants and children in their first five years of life, with the majority (86%) describing interventions for infants within their first three months of life (Figure 3). Of the 28 articles, 25 evaluated the integration of HIV services into child health platforms, while only three articles investigated the integration of child health services into HIV platforms. 20-22 The definition of “integration” and timeframe across the 28 articles also varied widely.
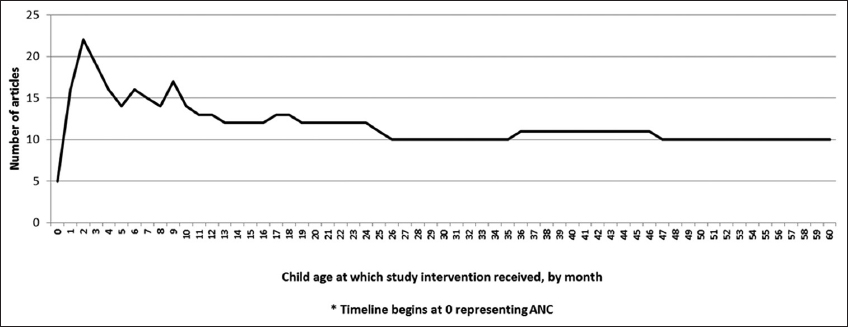
- Number of Articles and Age of Child Receiving Study Interventions
3.2. Outcomes of service integration
The primary outcomes described across the 28 studies were grouped into four categories: 1) Health outcomes of children as a result of integrated services, 2) Integration effects on service uptake, 3) acceptability of integrated services, and 4) enablers and barriers to service integration. Results in each of these categories are summarized below and in Tables 6-9.
3.2.1. Health outcomes of children
Nine articles assessed the health outcomes of children in the mother-child dyad when services were integrated. These articles described interventions within four main clinical platforms: nutrition services, pediatric inpatient wards, child survival programs and TB clinics (Table 6).
Four articles assessed the integration of HIV care and nutrition services, and found varied effects on child health outcomes. Two articles reported positive health outcomes when HIV services were integrated into nutrition services: Amadi et al.23 found a reduction in the child mortality rate due to acute malnutrition when a comprehensive community childhood malnutrition program incorporated HIV testing services. Over the three-year program, the authors noted a 0.5% mortality rate with moderate acute malnutrition (MAM) and a 4.2% mortality rate with severe acute malnutrition (SAM), which compared to concomitantly reported inpatient SAM mortality rates ranging from 3-35%.24 In another study, Kim et al. reported on the integration of HIV care and treatment, including HIV testing, ART, and opportunistic infection management, into an outpatient therapeutic feeding program.25 The authors found that prompt ART initiation within 21 days of diagnosis was associated with increased likelihood of nutritional recovery (OR: 5.4, 95% CI: 2.0 - 14.5). The two other studies assessed the integration of nutrition supplement services into HIV PMTCT programs and found variable effects on infant health outcomes.20,21 In a cohort of 2387 infants whose mothers had receive ART during pregnancy, Duggan et al. found that infants receiving micronutrient supplementation (n=1193) demonstrated a significant reduction in morbidities, including fewer hospitalizations (P = 0.035), episodes of fever (P = 0.005), and episodes of fever and cough (P =0.019) compared to a control group who did not receive micronutrient supplementation.20 In another study, Kindra et al. found no difference in weight gain between infants whose HIV-positive breastfeeding mothers had received a nutritional supplement paste and a control group.21 Reasons given for no difference between the groups was non-adherence due to stigma that mothers perceived being seen carrying the supplement package and the sweet taste of the supplement.
One study detailed health outcomes for children when HIV testing was integrated into pediatric inpatient wards. Preidis et al. found that routine hospital-based provider initiated testing and counseling (PITC) identified younger patients (median 25.0 vs 53.5 months) with more severe disease (22.2% vs 7.8% in clinical stage IV)9 compared with community-based testing. 26 In addition, more children identified in the hospital were eligible for ART based on age (47% vs 23% in community), and were on ART 12 months after diagnosis (91.4% vs 75.0%, p = 0.005). The mortality rate for hospital-identified children (15.5%) was double the mortality rate for community-identified children (7.8%) (p<0.0125).
Three articles found improvement in child outcomes when child survival programs and HIV PMTCT services were integrated. The first article described a comprehensive community-based home visit approach to maternal and newborn care for HIV-positive women and their infants.27 Women in the intervention arm received seven community health worker (CHW) home visits peri-partum to provide care consistent with ICMI, PMTCT, lactation counseling and newborn care guidelines, while control participants received three social welfare counseling visits without a health component. Prevalence of exclusive breastfeeding at 12 weeks increased among women receiving the intervention (29%) compared with control (15%) (RR 1.92), and there was a trend towards more infants in the intervention arm receiving a DNA-PCR test (73.6% vs 66.6%). No difference was seen between study arms in HIV-free infant survival.
Lilian et al. assessed the effect of an intervention providing early HIV testing (<6 weeks of age) in a postnatal care setting and found a modest effect on infant mortality, demonstrating a 16% mortality rate in the study population as compared to the national mortality rate of 20%.28 The third study described an integrated, comprehensive child survival program that incorporated HIV PMTCT care, demonstrating high rates of infant survival and retention in care.29 This intervention included access to home-based care, PMTCT services, clean water, nutrition support and socioeconomic support, and found that the probability of 18-month survival of HEI was 93% (95% CI: 0.91 to 0.94) and the infant retention rate in the same time period was 88% (95% CI: 0.86 to 0.90).
Patel et al. found a positive effect on child health outcomes from integration of HIV treatment services into a nurse-centered pediatric TB clinic, demonstrating greater weight gain among HIV-positive children receiving TB treatment who initiated ART compared to those who did not (2.2 vs. 0.7 kg/m2, P = 0.08). 30
3.2.2. Service uptake
Eighteen articles assessed the effect of integrated child health and HIV platforms on the uptake of those services by beneficiaries. Most of the articles used caregiver consent to services as a proxy for child consent. Services included HIV testing and immunization services, post-natal childhood survival services, provider initiated counseling and testing (PITC) in inpatient and outpatient wards, and nutrition programming (Table 7).
Five articles examined how integration affects the uptake of HIV testing and immunization services.31-36 To assess the effect of integration on HIV testing uptake, McCollum et al. compared the rate of consent by caregivers to infant HIV DNA-PCR testing at EPI clinics compared with at under-fives clinics, and found a 7-fold greater proportion of infants received the test at EPI clinics (84.2% vs 11.4%, p<0.001) than those being seen at the under-fives clinic.31 Not only was the proportion of children tested at the EPI clinic greater, but those children who received HIV DNA-PCR testing at this location were also 2.5 months younger (3.1 vs 5.6, p<0.001), and were about three times more likely to return for their DNA-PCR results and enroll in HEI care than those tested at the under-fives clinic (78.6% vs 25.0%, p<0.001). When opt-out HIV testing was integrated into EPI clinics, Wang et al. found that, compared to the control arm, the numbers of infants receiving testing in the intervention arm increased on average by 16.6% monthly (90% CI: -7%, 46%, p = 0.26) and increased in the “comprehensive” intervention arm on average by 10% monthly (90% CI: -10%, 36%, p = 0.43).31 Ong’ech et al. found that infants in their integrated “MCH model” were over 2 times more likely to receive testing for HIV antibodies at 1 year of age, than in the control (ART clinic) model (95% CI: 1.57-3.18).34 Patel et al. studied the effect of a community based play-center model on the uptake of HIV testing of children and found that just 59% (410/697) of the enrolled children were tested, but all 74 children found HIV positive were successfully initiated on ART.36
Levin et al. observed a lower uptake of testing for infants among mothers of unknown or undisclosed status (8/24; 33.3%) compared with a 77% uptake of infant testing among known HIV-positive mothers (83/107; 77.6%), when offering HIV testing for infants at their 18 month immunization appointment.35 Rollins et al. found that when HIV testing was offered at the EPI clinic, 90% of mothers attending agreed to HIV testing of their infants, yet only 57% returned for results.37
When assessing the effect of integration of HIV services on immunization uptake, Wang et al. found that integrating HIV testing services with infants immunization services at under-five clinics did not have a significant effect on DPT1 immunization uptake, yielding an average of 0.86 more doses of DPT1 per month compared to facilities that did not receive the intervention.33 Goodson et al., however, found variations between vaccines administered in rural and urban sites after early infant diagnostic (EID) HIV testing was integrated. At the urban sites, doses for later age vaccines increased (pentavalent, polio and measles up 12%, 8% and 11%) while in rural sites, vaccine doses given by the first month decreased by 33% and 35%, and vaccine doses given later in life decreased by 23%, 28%, and 28% respectively, compared to baseline at rural clinics.32 Ong’ech et al. found that infants in the integrated “MCH model” arm were 1.14 times (95% CI: 1.04 to 1.26) more likely to attend the 14-week immunization visit.34 Patel also found that 90% of children enrolled in the community based play-center model were fully immunized (n=629), compared with the national coverage estimates of 75%.36 Levin and McCollum (2012) did not include an assessment of the effect of integration on immunization service uptake.
Six articles found that postnatal follow-up with integrated HIV and childhood survival interventions increased service uptake.27,29,38-41 Mazia et al. observed that integrating PMTCT services into postnatal care led to a 20-fold increase in the number of mothers returning for a post-natal visit within 3 days of the birth; breastfeeding by HIV-positive mothers increased by 41%; and provision of cotrimoxazole prophylaxis for HIV-exposed infants increased by 24%.42 Similarly, Tomlinson et al. found that mothers who received more frequent CHW home visits demonstrated double the rate of exclusive breastfeeding (RR1.92; 95% CI: 1.59–2.33); an increase in the number of infants with an HIV test by six weeks of age (67% vs. 74%, RR1.10); and higher cotrimoxazole uptake at 12 weeks postpartum (37% vs. 43%, RR 1.17, 95% CI: 0.99–1.37).27 Gupta observed that a comprehensive package of HIV-free and child survival services led to improved uptake of contraception and treatment for diarrheal disease.29
Three articles reported on the effect of integration of antenatal care (ANC) and PMTCT services.39-41 Herlihy found that integrating ANC and PMTCT services resulted in improved six-week dried blood spot (DBS) DNA-PCR results of 55.8% from a baseline of 41.9% (P< 0.01).40 When providing ART at ANC/PMTCT sites, Turan noted that HIV care enrollment was higher in the intervention sites compared to control sites (69% versus 36% [OR=3.94, 95% CI: 1.14–13.63]), and time to HIV care enrollment was significantly shorter among women in the intervention arm (0 versus 8 days [HR =2.20, 95% CI: 1.62–3.01]).39 Finally, in decentralizing PMTCT services from HIV care and treatment sites to ANC/Labor and delivery (L&D) wards, Edmonds et al. noted that a greater absolute number of mothers were reached, however ANC/L&D wards showed poorer provision of PMTCT services (pregnancy package of services by 14 weeks 88% vs 66%; delivery of CTX and EID 97% vs 89%) than was observed in the HIV care and treatment sites.41
Four articles that explored the integration of PITC into inpatient and outpatient wards also described the positive effects of integration on the number of caregivers or children tested.43-46 Wanyenze et al. observed that 92% (n=8,990) of caregivers consented for their children to receive HIV testing in pediatric wards over a year, and the majority of these children (8663, 96.0%) had never been previously tested.43 The authors found that HIV seroprevalence among children was 12.4% on average and highest on the nutrition ward (30.8%). Substantially increased uptake of HIV testing from 40.8% to 98.5% by eligible inpatient children was also observed by Mutanga et al.44 McCollum et al. (2011) found a greater proportion of all hospitalized children received HIV testing (81.0% vs 33.3%, p < 0.001), however PITC did not improve linkage of those identified as positive to ART, although greater than 85% were ART-eligible.45 Weigel et al. found that following introduction of PITC and ART availability to inpatient pediatric wards, the median number of children per quarter accessing HIV testing (101 to 358), registration (56 to 226) and ART initiation (18 to 139) increased, although providers initiated testing in less than 10% of admitted children.46
Amadi et al. studied the integration of HIV testing into a Community Management of Acute Malnutrition program and found 97% of enrolled children were tested for HIV, with a positivity rate of 10%. In addition, 66% of identified positive children were initiated on ART.23
3.2.3. Acceptability of integrated services
Acceptability of integrated services was frequently cited in many of the articles, yet definitions of acceptability ranged from service uptake proxies to client and provider perceptions. To identify studies reporting acceptability of integrated services, we defined acceptability per Sekhon et al. as “a multi-faceted construct that reflects the extent to which people delivering or receiving a healthcare intervention consider it to be appropriate, based on anticipated or experienced cognitive and emotional responses to the intervention”.47 Using these criteria, we determined that eight articles reported on the acceptability of integrated services to patients, caregivers, or providers through interviews or focus group discussions, many of which were qualitative or semi-quantitative. The findings for these articles are summarized in Table 8.
While most articles (n=6) assessed caregiver and provider acceptability of the integration of HIV testing services (HTS) into routine child health services, two articles addressed acceptability of non-HTS services.21,38 Through interviews with HIV-positive mothers to understand non-adherence to a nutritional supplement paste provided at ANC, mothers reported the poor taste of the supplement and stigma associated with carrying a package from the clinic.21 In an intervention where providers received additional training on standard postnatal care services to improve the quality of PMTCT services, end line interviews found that mothers perceived an improvement in the quality of care and demonstrated increased knowledge and practices of post-partum and infant care.38
Of the six articles reporting on acceptability of HTS integration with child health services, five found that integrating HTS into primary healthcare settings, including routine consultation or immunization visits, was perceived as acceptable and beneficial to both caregivers and providers. Interviews with mothers revealed a range of reasons why mothers found HTS acceptable, including: supporting the child’s health; allowing for early ART initiation; informing feeding practices; protecting the mother and her family; and reducing child mortality.22,32,48 Similar results were also seen from focus group discussions in under-five clinics where mothers revealed positive attitudes to HTS and perceptions of benefits to their children’s well-being as well as their own health. In this study, no evidence was found to indicate caregivers would be less inclined to attend an under-five clinic to avoid HIV testing.33
In three articles, healthcare providers gave positive feedback during interviews on the benefits of integrated services for infants and mothers, including: increased identification of HIV-infected infants; opportunity to start infants on treatment; and saving mothers travel time and money.37,48 Staff who received extra training when PITC services were integrated into all pediatric entry points in a large hospital reported that HTS was easier to conduct for several reasons, including: the clinician could introduce the topic of HIV earlier with the client; clients were more willing to test knowing that ART was available on-site; and that provider training had reduced stigma directed towards clients for accessing HTS.44
Three articles identified caregiver or provider concerns over HIV testing as a barrier to acceptability of integrated services. At an immunization clinic, some mothers who chose not to receive testing or to allow their child to be tested on the same day of service were frightened that testing their child would reveal their own HIV status.37 Other mothers at an immunization clinic reported the misconception that HTS was required to receive immunizations, and expressed concerns over privacy and extended wait times due to integrated services.32 Despite accepting the principle of routine infant HIV testing at postnatal points of care in community health centers, Ndondoki et al. found that 61% of mothers did not return with their infant for the testing procedure.22 When interviewed, mothers gave reasons including needing partners consent; concerns of the stigma of their child testing positive, or self-stigmatization, concerns also expressed by mothers interviewed by Mutanga et al.44
3.2.4. Enablers and barriers to Service Integration
Fourteen articles discussed health system enablers and/or barriers to service integration (Table 9). Enablers that contributed to the successful integration of services centered on issues of human resource deployment and capacity, quality of services, and tools for data collection. Several articles (n=3) found that improved training of providers prior to the launch of interventions equipped them with enhanced knowledge and improved service delivery and customer service skills.37,38,46 Four articles discussed other proactive approaches to address human resources needs, including re-deployment of existing staff,44,49 hiring more staff,43 and task shifting by assigning tasks to nurses, or increasing the use of lay cadres at sites or in the community to support care coordination.30,44 One article described the success of a quality improvement approach through provider mentoring, regular supervision sessions, and spot checks of performance, as well as regular staff meetings to assess performance of PITC integration.46 Where HIV care and treatment services were integrated, one study reported that the use of low tech tools that were regularly reviewed by providers and health facility managers, such as registers, allowed regular performance review and contributed to the success of service integration.41
Barriers to successful integration identified in the articles included inadequate staffing, clinic space factors, and external health system-related barriers. Several articles reported challenges with staffing of integrated interventions, ranging from increased demand on personnel and high government mandated training fees,43,45 to insufficient staffing footprint at clinics that reduced the time providers dedicated to providing integrated services.48 Horwood et al. cited that roles and responsibilities were not well-defined between providers of integrated ANC-PMTCT services, and record keeping on what services had been received by mothers and infants was poor.50 Physical space factors were also cited, including lack of confidential testing and counseling spaces in clinics and poor patient flow.37,48 Several articles cited external health systems factors that acted as barriers to integrated services, including concerns over stock levels of HIV test kits and turnaround time for PCR results.33,44,49
4. Discussion
Multiple global bodies have described integration of child health and HIV interventions as an approach to improve health outcomes and service delivery and to increase technical and financial efficiencies of health programs.51,52 Anecdotally, many countries are integrating services; however, understanding the scope of this integration, and measuring the quality, effect, acceptability, and feasibility of integrated services has been challenging as the evidence base is not extensive. In this review, we mapped studies of integrated services to four outcome categories in order to provide an improved evidence base to inform integration of child health and HIV service programs.
A key argument in support of integrating child health and HIV services is that doing so will improve health outcomes and service uptake, an idea supported by this review’s findings. In this review, HIV services were mainly integrated into maternal and child health platforms, a reflection of long-standing national child survival programs and the urgent need to identify HIV-positive infants and children. The need for best practices in HIV case identification is reflected by the large number of reviewed articles (16/28) that assessed integration of HIV testing services into various locations within the facility—from the EPI clinic, to inpatient wards, to TB and malnutrition services and under 5 clinics. For the majority of these studies, the uptake of HIV testing was high in the integrated programs, showing that integrating HIV testing into other service platforms identified numerous HIV-infected children that may not have otherwise been identified by the standard of care.
Results from this review show that integration of HIV testing services into child health platforms increases the number of infants and children living with HIV identified, the first step to getting them access to ART and preventing under five mortality. In trying to explain what fuels the successes of the integrated interventions, the authors posited that systematization of combined services, such as retraining of staff in key child survival activities and use of opt-out HIV testing services, could be drivers of success.27,29,36-40,44,46,49 In addition to systematization, the authors note that staffing adjustments might also have assisted in improving service outcomes and uptake. These studies either had additional staff funded through the study, or incorporated sharing of responsibilities between the various facility cadres, thereby apportioning the workload of the added services being provided. 27,29,31,43-45,49
A key concern of programmers is that integration could have a negative effect on the quality of service delivery in the existing service delivery platform. In this review, the authors looked carefully at this question and noted that the articles that assessed the effect of integration on the base platform showed either a positive or a neutral effect on the base services, with just one noting a negative effect. Examples of positive and neutral findings include: an increased uptake of postnatal services38 and a doubling of exclusive breastfeeding rates27 when HIV services were integrated into ANC services; high rates of tuberculosis treatment completion by children and increased weight gain during TB therapy30 after HIV testing was integrated into TB programs; and either no effect on immunization uptake33 or a higher proportion of vaccines administered in the older age groups when HIV testing was incorporated.32 One article that also showed a negative effect of service integration saw this effect when HIV testing was integrated into rural EPI clinics.32 When integration occurred at these facilities, significant declines were seen in immunization uptake—a significant difference from outcomes observed in urban facilities involved in the same study. The study authors identified that the decline in immunization uptake resulted from clients’ stigma and discrimination concerns, concerns that were amplified in rural settings as compared to urban ones. Any cause of reduction in service uptake should be carefully addressed.
While many of the reviewed studies show that integration is acceptable by both clients and providers, for national scale integration to be feasible, policy makers and service providers need to carefully consider the enablers and barriers identified in this review. The results highlight that successful scale-up of integrated child and HIV platforms requires up-front investment in the health workforce, training, and on-site mentoring as well as careful considerations of patient flow, clinic organization and confidentiality of treatment spaces, and appropriate technology. Feasible scale-up also hinges on effective supply chain and laboratory systems.
It is clear from the reviewed studies that issues of fear, stigma, privacy, and potential for discrimination are still significant determinants of patients’ decisions around accessing HIV services for themselves and their children. As seen in the findings reported from six articles, there is a potential concerns involved when integrating HIV testing into other maternal and child health services, if confidentiality, stigma and discrimination issues are not addressed.21,32,37,39,44,48 A good communications strategy and proactive efforts to address stigma and confidentiality with both health care providers and beneficiaries is essential to effective integration. The stigma and discrimination concerns associated with HIV must be carefully and continually addressed, and the importance of context should not be underestimated.
When global health policymakers (e.g. Ministries of Health, program planners, and funders) consider integration of child health and HIV services for efficiency, each clinical “touch-point” with infants and children can be leveraged to provide comprehensive health services (Figure 1). As a first step towards integration, global health policymakers should carefully review country specific national and sub-national health indicator data to identify barriers and opportunities for integrated service delivery platforms. Integration plans can be tailored to increase uptake or address gaps in specific services, to address potential health system barriers, and to leverage program strengths.
5. Conclusions and Global Health Implications
In sub-Saharan Africa, progress on reducing preventable infant and child deaths and increasing antiretroviral treatment (ART) coverage among CLHIV has been slower than desired and poses a significant challenge to global health. Interventions that integrate child health and HIV services have the potential to improve health outcomes by addressing multiple family health care needs simultaneously and reducing missed opportunities to reach infants and children with all of the health services they require. In the current era of flat funding levels, scale-up of treatment for CLHIV, and increasing access to infant and child mortality reduction interventions, the strategic integration of services should be a key focus of health system policy makers, program planners, funders, and service providers.
The findings of this review build a clear evidence base showing that integration of HIV and child health services can be an effective way to synergize existing health platforms, and successfully achieve positive outcomes in all interventions. In the majority of articles included in this review, integration was found to benefit, or at least have no negative effect on, HIV and child health outcomes. This systematic review has shown that integration is acceptable to patients and providers; that larger numbers of infants and children can be reached through integration; and that for integration to succeed, proactive steps should be taken to ready the health system to integrate at scale.
5.1. Limitations
Several limitations were noted throughout this literature review. First, several articles (n=14) did not have comparator groups, such as control groups or national or local estimates, challenging the review of causation between integrated services and outcomes.22,23,25,28-30,35,36,43,44,48-50 Second, two of the articles that reviewed HIV service integration into immunization platforms only reported on the effect on HIV services and did not examine or report on the effect of integration on immunization uptake.31,35 Future research should consider including a study of the effect on all services and platforms included in the integrated package. Third, as stated in the methodology section and in Table 3, 11 of the reviewed studies were of fair quality and four were of poor quality. Since quality review was not used to eliminate articles from the review, some of the data and conclusions drawn are based on less than optimal study designs.
Given the existing body of evidence on the benefits of integrating child health and HIV services and its positive perceptions by clients and providers alike, further research should be centered on implementation science or operational research as countries move toward integration at scale. With the emphasis on Test and Start, future studies should focus on the gaps in linkage from diagnosis of HIV to treatment, and explore how to address the valid concerns of families and providers.
During integration, robust monitoring and analysis of integrated child health and HIV service platforms can further drive the development of optimized health services. The research evidence shows that investments in integration have the potential to produce significant results and improve overall infant and child health. Taken together, these strategies can advance progress towards the global goals of ending preventable infant and child deaths and gaining control of the HIV/AIDS epidemic.
Supplementary
Acknowledgements
The views and opinions expressed in the article are solely those of the authors and do not necessarily reflect those of the US Agency for International Development nor those of the US Government. We gratefully acknowledge Allison Ficht and Henry Miller for their support in collecting the data used in this manuscript.
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethics Approval: This study was based on analysis of existing published studies.
Funding: Publication costs for this study were covered by the Global Health Fellows Program-II, Washington, DC to BS.
References
- Levels and Trends in Child Mortality - Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation 2017
- Neonatal mortality and coverage of essential newborn interventions 2010 - 2013:a prospective, population-based study from low-middle income countries. Reproductive health. 2015;12(Suppl 2):S6.
- [Google Scholar]
- HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa:a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103(6):541-548.
- [Google Scholar]
- Severe morbidity and mortality in untreated HIV-infected children in a paediatric care programme in Abidjan, Cote d'Ivoire, 2004-2009. BMC Infect Dis. 2011;11:182.
- [Google Scholar]
- Effect of maternal HIV status on infant mortality:evidence from a 9-month follow-up of mothers and their infants in Zimbabwe. Journal of perinatology:official journal of the California Perinatal Association. 2010;30(2):88-92.
- [Google Scholar]
- Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. Aids. 2009;23(1):101-106.
- [Google Scholar]
- Child mortality and HIV infection in Africa:a review. Aids. 2004;18(Suppl 2):S27-34.
- [Google Scholar]
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization 2016
- [Google Scholar]
- On the Fast-Track to an AIDS-free generation:The final report of the Global Plan 2016
- Regional Strategic Plan for Immunization 2015-2020. WHO Regional Office for Africa 2015
- Start Free, Stay Free, AIDS Free 2016
- Preventing HIV during pregnancy and breastfeeding in the context of PrEP 2017
- Integration of HIV in child survival platforms:a novel programmatic pathway towards the 90–90–90 targets. Journal of the International AIDS Society. 2015;18(7 Suppl 6):20250.
- [Google Scholar]
- Integrated Health Services Technical Brief 2008
- Integrated care:meaning, logic, applications, and implications--a discussion paper. International journal of integrated care. 2002;2:e12.
- [Google Scholar]
- The Double Dividend 2013
- Integrated Management of Childhood Illness (IMCI) global survey report 2017
- Does integration of HIV and SRH services achieve economies of scale and scope in practice? A cost function analysis of the Integra Initiative. Sex Transm Infect. 2016;92(2):130-134.
- [Google Scholar]
- Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers:a randomized, double-blind, placebo-controlled clinical trial. Am J Clin Nutr. 2012;96:1437-1446.
- [Google Scholar]
- Effect of nutritional supplementation of breastfeeding HIV positive mothers on maternal and child health:findings from a randomized controlled clinical trial. BMC Public Health. 2011;11:946.
- [Google Scholar]
- Universal HIV screening at postnatal points of care:which public health approach for early infant diagnosis in Cote d'Ivoire? PloS One. 2013;8(8):e67996.
- [Google Scholar]
- Integration of HIV Care into Community Management of Acute Childhood Malnutrition Permits Good Outcomes:Retrospective Analysis of Three Years of a Programme in Lusaka. PLoS One. 2016;11(3):e0149218.
- [Google Scholar]
- Treatment of severe and moderate acute malnutrition in low- and middle-income settings:a systematic review, meta-analysis and Delphi process. BMC Public Health. 2013;13(Suppl 3):S23.
- [Google Scholar]
- Prompt initiation of ART with therapeutic food is associated with improved outcomes in HIV-infected Malawian children with malnutrition. J Acquir Immune Defic Syndr. 2012;59(2):173-176.
- [Google Scholar]
- Routine inpatient provider-initiated HIV testing in Malawi, compared with client-initiated community-based testing, identifies younger children at higher risk of early mortality. J Acquir Immune Defic Syndr. 2013;63(1):e16-e22.
- [Google Scholar]
- Goodstart:a cluster randomised effectiveness trial of an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township. Trop Med Int Health. 2014;19(3):256-266.
- [Google Scholar]
- Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J. 2013;32(10):1080.
- [Google Scholar]
- Clinical outcomes of a comprehensive integrated program for HIV-exposed infants:a 3-year experience promoting HIV-free survival in rural Rwanda. J Acquir Immune Defic Syndr. 2013;62(4):e109-e114.
- [Google Scholar]
- Outcomes of integrated treatment for tuberculosis and HIV in children at the primary health care level. Int J Tuberc Lung Dis. 2013;17(9):1206-1211.
- [Google Scholar]
- Superior uptake and outcomes of early infant diagnosis of HIV services at an immunization clinic versus an “under-five”general pediatric clinic in Malawi. J Acquir Immune Defic Syndr. 2012;60(4):e107-e110.
- [Google Scholar]
- Evaluation of using routine infant immunization visits to identify and follow-up HIV-exposed infants and their mothers in Tanzania. J Acquir Immune Defic Syndr. 2013;63(1):e9-e15.
- [Google Scholar]
- A Cluster Randomised Trial on the Impact of Integrating Early Infant HIV Diagnosis with the Expanded Programme on Immunization on Immunization and HIV Testing Rates in Rural Health Facilities in Southern Zambia. PloS One. 2015;10(10):e0141455.
- [Google Scholar]
- Provision of services and care for HIV-exposed infants:a comparison of maternal and child health clinic and HIV comprehensive care clinic models. J Acquir Immune Defic Syndr. 2012;61(1):83-89.
- [Google Scholar]
- Acceptability, feasibility and impact of routine screening to detect undiagnosed HIV infection in 17 - 24-month-old children in the western sub-district of Cape Town. South African Medical Journal. 2012;102(4):245.
- [Google Scholar]
- Facilitating HIV testing, care and treatment for orphans and vulnerable children aged five years and younger through community-based early childhood development playcentres in rural Zimbabwe. Journal Of The International AIDS Society. 2012;15(Suppl 2):17404.
- [Google Scholar]
- Universal HIV testing of infants at immunization clinics:an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851-1857.
- [Google Scholar]
- Integrating quality postnatal care into PMTCT in Swaziland. Global Public Health. 2009;4(3):253-270.
- [Google Scholar]
- Implementation and Operational Research:Effects of Antenatal Care and HIV Treatment Integration on Elements of the PMTCT Cascade:Results From the SHAIP Cluster-Randomized Controlled Trial in Kenya. Journal Of Acquired Immune Deficiency Syndromes. 2015;69(5):e172-e181.
- [Google Scholar]
- Implementation and Operational Research:Integration of PMTCT and Antenatal Services Improves Combination Antiretroviral Therapy Uptake for HIV-Positive Pregnant Women in Southern Zambia:A Prototype for Option B+? J Acquir Immune Defic Syndr. 2015;70(4):e123-e129.
- [Google Scholar]
- Implementation and Operational Research:Decentralization Does Not Assure Optimal Delivery of PMTCT and HIV-Exposed Infant Services in a Low Prevalence Setting. J Acquir Immune Defic Syndr. 2016;70(4):e130-e139.
- [Google Scholar]
- Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care:a randomised controlled trial. The lancet HIV. 2016;3(2):e85-93.
- [Google Scholar]
- Provider-initiated HIV testing for paediatric inpatients and their caretakers is feasible and acceptable. Trop Med Int Health. 2009;15(1):113-119.
- [Google Scholar]
- Institutionalizing provider-initiated HIV testing and counselling for children:an observational case study from Zambia. PLoS One. 2012;7(4):e29656.
- [Google Scholar]
- Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30(5):e75-e81.
- [Google Scholar]
- Effect of provider-initiated testing and counselling and integration of ART services on access to HIV diagnosis and treatment for children in Lilongwe, Malawi:a pre- post comparison. BMC Pediatrics. 2009;9(80)
- [Google Scholar]
- Acceptability of healthcare interventions:an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17(1):88.
- [Google Scholar]
- Qualitative assessment of the integration of HIV services with infant routine immunization visits in Tanzania. J Acquir Immune Defic Syndr. 2014;66(1):e8-e14.
- [Google Scholar]
- Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia:acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51(2):202-208.
- [Google Scholar]
- Prevention of mother to child transmission of HIV (PMTCT) programme in KwaZulu-Natal, South Africa:an evaluation of PMTCT implementation and integration into routine maternal, child and women's health services. Trop Med Int Health. 2010;15(9):992-999.
- [Google Scholar]
- The State of the World's Children 2016 2016
- Integrated Delivery:Strategy Overview 2018 https://www.gatesfoundation.org/What-We-Do/Global-Development/Integrated-Delivery





