Translate this page into:
Introduction of Heat-Stable Carbetocin through a Public-Private Partnership Model in India: A Retrospective Study to Determine Feasibility and Its Optimal Use in Public Health Settings

*Corresponding author: Dr. Harish Kumar, Project Director, SAMVEG: ‘Systems Approach for MNCH focusing on Vulnerable Geographies’, Supported by USAID, IPE Global Ltd, B-84 Defence Colony, New Delhi, India. Tel: +91 11 40755900, harishkumar@ipeglobal.com
-
Received: ,
Accepted: ,
How to cite this article: Alwadhi V, Mishra A, Nagendra A, Bajpayee D, Joshi NC, Gupta S, et al. Introduction of heat-stable carbetocin through a public-private partnership model: A retrospective study to determine feasibility and optimal use in public health settings in India. Int J MCH AIDS. 2024;13:S81-88. doi: 10.25259/IJMA_11_2023
Abstract
Background and Objective
The optimal use of oxytocin for preventing postpartum hemorrhage (PPH) faces challenges in many low-middle income countries (LMICs) owing to its storage and transportation prerequisites. We demonstrated Heat-Stable Carbetocin (HSC) for PPH prevention through an innovative Public-Private Partnership (PPP) model in 15 public health facilities of the Dewas District of Madhya Pradesh (MP) state in India. This study evaluates the feasibility and appropriate utilization of HSC in public health settings.
Methods
We analyzed facility-level data collected between August 2022 and July 2023 from selected 15 health facilities, where HSC was introduced. Prior to the introduction of HSC, all healthcare providers received training on Active Management of the Third Stage of Labor (AMTSL), use of HSC, and recording and reporting procedures. The supply of HSC in health facilities was ensured, and a robust mechanism was set up to monitor the progress.
Results
A total of 18,497 women were admitted for delivery in the 15 selected facilities. Uterotonic administration within one minute of delivery was almost universal (99.9%). No instance was recorded of using HSC either for induction of labor or management of PPH. In 636 cases (3.43%), HSC was not given for PPH prevention. Pearson’s chi-square test was conducted to assess the relationship between HSC usage and the health facility’s level. The HSC use was significantly higher in First Referral Unit (FRU) facilities compared to non-FRUs (p < 0.001). Moreover, the administration of HSC within one minute of delivery was also more prevalent in FRU facilities compared to non-FRUs (p < 0.001). The PPH incidence and case referral rates noted in this study were 0.7% and 16.7%, respectively, with no reported adverse drug events or deaths.
Conclusion and Global Health Implications
Our study suggests the safe and appropriate use of HSC within India’s public health system.
Keywords
Postpartum Hemorrhage
Heat-Stable Carbetocin
Uterotonic
Active Management of Third Stage of Labor
Maternal Health
INTRODUCTION
Background of the Study
Childbirth is the most crucial time when pregnant women and newborn babies are at the highest risk of morbidity and mortality. Nearly 46% of all maternal deaths and 40% of neonatal deaths usually occur during labor or the first 24 hours after birth. Postpartum hemorrhage (PPH), hypertension, sepsis, and obstructed labor are the major causes of maternal deaths.[1] Most of these deaths can be prevented by conducting evidence-based practices during the period around childbirth.
The PPH is a leading cause of maternal death globally, accounting for about one-quarter of all maternal deaths. India, with its high maternal mortality rate (97 deaths per 10,000 live births from 2018 to 2020)[2] is no exception, as 38% of maternal deaths are due to PPH.[3]
Oxytocin, a World Health Organization (WHO) recommended uterotonic drug is one of the most widely used drugs for preventing and managing PPH. Oxytocin has a short half-life of 1–6 minutes,[4] making it a suitable drug for safely inducing or augmenting labor in specific clinical scenarios.[5] However, oxytocin is not without its challenges. Oxytocin is heat-sensitive and must be stored at 2–8 degrees Celsius,[4] which is difficult to maintain in many low- and middle-income countries (LMICs) like India because of their insufficient cold chain capacity. As a result, studies have shown that much of the oxytocin available in LMIC markets like India either does not meet drug quality standards or is stored improperly.[6–8]
In response to global interest in developing a heat-stable alternative to oxytocin for preventing PPH, a new drug, Heat-Stable Carbetocin (HSC), has been shown to prevent PPH effectively.[9–11] Unlike oxytocin, HSC is stable at 30 degrees Celsius, which is advantageous in areas without adequate cold chain capacity.[9] This drug is new to the market and has been registered recently in India, and injectable HSC (100 μg/mL) was added to the WHO’s Model List of Essential Medicines in 2021.[12] At the same time, WHO has recommended HSC in contexts where the quality of oxytocin cannot be guaranteed.[4] HSC is contraindicated for inducing and augmenting labor due to its long half-life of 40 minutes.[4,5] Oxytocin and HSC pose unique challenges, particularly in India, where misuse of oxytocin is rampant and there is a risk of inappropriate HSC use.[13]
A recent study from India showed that HSC prevents more disability-adjusted life years (DALYs) and reduces costs to the public healthcare system of India.[14] In 2020, the Drug Controller General of India (DGCI) approved the use of HSC for preventing PPH, in alignment with WHO recommendations. However, no studies have been conducted so far to test the feasibility and optimal use of HSC in the existing public health system. Further research is imperative to ensure the safe and appropriate utilization of HSC to guide policymakers before its widespread adoption as an alternative to oxytocin for PPH prevention, particularly in settings where oxytocin quality may be questionable.
Under the leadership of the National Health Mission (NHM) of the State of Madhya Pradesh (MP) in India, a public-private partnership (PPP) was forged through the United States Agency for International Development’s (USAID) India-awarded Systems Approach for Maternal, Newborn, and Child Health (MNCH) focusing on Vulnerable Geographies (SAMVEG) project. A consortium partnership was formed, including IPE Global Limited, DIMAGI, Inc., World Health Partners, and John Snow India Private Limited, to demonstrate an innovative model using HSC as an entry point for strengthening active management of the third stage of labor (AMTSL). Ferring Pharmaceutical, the manufacturer of HSC, was also engaged in selected health facilities for its commitment to supply HSC. The key steps involved in the development and implementation of HSC model is presented as Figure 1.
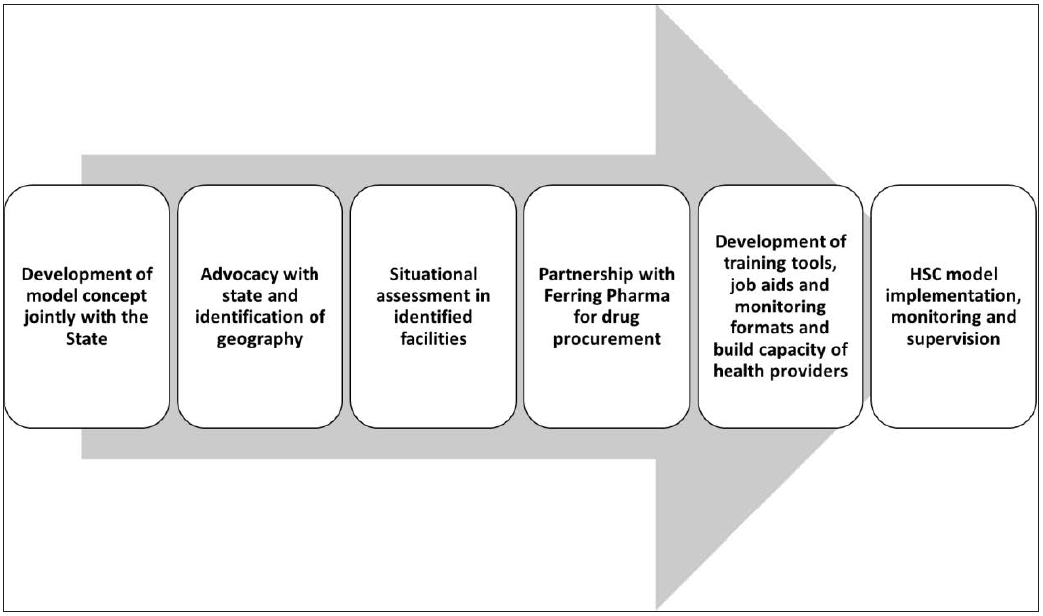
- Key steps involved in the development and implementation of the Heat-stable carbetocin (HSC) model.
Study Objectives
The objectives of the study were to (1) understand the feasibility of introducing HSC through an innovative PPP model in the First Referral Unit (FRU) and non-FRU-level public health facilities; (2) understand the clinical practices for prophylactic use of HSC; and (3) understand the factors that enable the appropriate use of uterotonics in public health settings.
Specific Aim
The study aimed to assess the acceptability and feasibility of introducing the HSC through a PPP model, ascertain its appropriate use, and derive learnings for its possible scale-up across similar settings. Additionally, the study also aimed to examine the key factors that facilitate or restrict the appropriate utilization of HSC in public health systems, as per WHO recommendations.
Methods
This manuscript followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) recommendations for the reporting of study findings. Fifteen public health facilities out of 32 in the Dewas District of MP were purposively selected, based on the monthly delivery load and their ability to provide comprehensive emergency obstetric and newborn care (CEmONC) services to demonstrate the implementation of this innovative model. A detailed situational analysis of health facilities was conducted to assess the status of AMTSL practices, availability of human resources, training, uterotonics, cold chain processes, and recording and reporting of service data. A specially constituted Technical Advisory Group (TAG) developed the training materials and job aids per the existing country guidelines, focusing on the appropriate use of HSC, including contraindications to its administration. Local language adaptation was done after obtaining State Government approvals for the training materials. Based on the findings of the situational analysis, one-day training on strengthening care around AMTSL, including appropriate use of HSC, was provided to healthcare providers, and the provision of posters and job aids was also ensured for a practical and standardized implementation of the model. The supply of HSC in all the selected health facilities was secured, and a robust monitoring and supportive supervision mechanism was set up to monitor the project’s progress.
Study Design and Settings
This was a retrospective study wherein we analyzed facility-level data collected between August 2022 and July 2023 from the 15 selected health facilities in the Dewas district of MP, where HSC was introduced through a PPP model. The health facilities comprised two FRU-level facilities, that is, one district hospital and one community health center (CHC), along with 13 non-FRU facilities: three CHCs, eight Primary Health Centers (PHCs), and two Health and Wellness Centers (HWCs).
Data Collection
At the facility level, usually a standardized paper-based delivery register and a case sheet are used for each delivery. Based on the findings of the situational analysis, new fields in the case sheet and delivery register were added to record the timing of uterotonic administration (within one minute of delivery). To ensure data accuracy, all service providers received training on AMTSL and the appropriate use of HSC as well as recording and reporting procedures before initiating the intervention. HSC was provided monthly using the state drug distribution system, and existing recording formats were adapted to include the uterotonic administration time. A nurse mentor provided supportive supervision and collected information related to the model once every month from each of the 15 facilities.
At the end of each month, facility staff gathers facility-level data from a paper-based delivery register. This data is then compiled using Microsoft Excel (Office 365) before being shared with the district nodal person based at the NHM for district-level compilation and analysis.
The monthly facility-wise delivery data compiled between August 2022 and July 2023 from all 15 health facilities were used to perform the analysis in this study.
Study Variables
The key outcomes of interest included: (1) the number of deliveries conducted in the facilities, deliveries where HSC was used, (2) the number of cases of HSC administration within one minute of delivery, (3) cesarean section deliveries that received HSC, and (4) patients given uterotonics for PPH management disaggregated by drug type. No personal identifiers of the beneficiaries delivered to health facilities were collected. Complete data analysis and interpretation were done in an aggregated form.
Statistical Analysis
We performed health facility-level analysis using Microsoft Excel (Office 365), and the results are reported in aggregated form in terms of frequencies and proportions. Additionally, the association between HSC use for PPH prevention and level of health facility was calculated and compared for statistical significance using Pearson’s chi-square test.
Ethical Approval
We received administrative authorization from the NHM of Madhya Pradesh before conducting this analysis. Additionally, this study was reviewed and approved by the International Institute of Health Management Research (IIHMR), New Delhi.
Results
The model was implemented in 15 of the existing 32 facilities providing delivery services, including two facilities providing CEmONC services, where 80% of deliveries were reported in the previous year. The project activities are currently ongoing. However, we analyzed 12 months of project implementation data of the study covering the period from August 2022 to July 2023.
Main Variable Results
A total of 88 service providers, including 70 nursing officers, were trained by leveraging the state government’s logistics and trainers. Implementation one year after the model’s launch by the State Government and supply of HSC revealed that, as per the records of the delivery point, a total of 18,497 women were admitted for care at childbirth in the 15 implementing facilities. In addition, 543 pregnant women (2.9%) who had transit deliveries before they could reach the delivery points were also managed. However, these were not included in the analysis.
Uterotonic administration within one minute of delivery was almost universal (98.8%). No instance was recorded of using HSC either for induction of labor or management of PPH. Facility-wise uterotonic administration and mode of delivery are shown in Table 1.
| Practices | Type of Facility | TOTAL | |
|---|---|---|---|
| FRU | non-FRU | ||
| Number of facilities | 2 | 13 | 15 |
| Total Deliveries | 11,536 | 6961 | 18,497 |
| Vaginal Delivery | 8270 (71.7%) | 6961 (100%) | 15,231 (82.3%) |
| Caesarean Delivery | 3266 (28.3%) | 0 | 3266 (17.7%) |
| Uterotonic used | 11,529 (99.9%) | 6952 (99.4%) | 18,481 (99.9%) |
| Uterotonic used within one minute of delivery | 11,523 (99.9%) | 6745 (96.9%) | 18,268 (98.8%) |
| HSC Used | 11,289 (97.9%) | 6572 (94.4%) | 17,861 (96.6%) |
| Oxytocin Used | 240 (2.1%) | 380 (5.5%) | 620 (3.4%) |
| HSC administered within one minute of delivery |
11,283 (99.9%) N = 11,289 |
6365 (96.9%) N = 6572 |
17,648 (98.8%) N = 17861 |
| Oxytocin administered within one minute of delivery |
223 (92.9%) N = 240 |
316 (83.2%) N = 380 |
539 (86.9%) N = 620 |
FRU: First referral unit, HSC: Heat-stable carbetocin, N: Number of cases wherein HSC was used.
In 636 cases (3.43%), HSC was not given for PPH prevention. PPH incidence documented in this study was 0.7%, with 126 reported PPH cases. A higher incidence was reported from non-FRU facilities (1.4%), and no PPH was reported in cesarean births. Uterine atony (69%) was the most reported cause of PPH, followed by birth trauma (23.8%). No case was reported where a surgical intervention was undertaken. At the same time, overall compliance with evidence-based monitoring practices with partograph (95.3%) and early initiation of breastfeeding (90.6%) was seen. The referral rate in PPH cases was 16.7%, and no death was reported, as shown in Table 2.
| PPH | Type of Facility | Total | |
|---|---|---|---|
| FRU | non-FRU | ||
|
Number of cases HSC Used Oxytocin Used |
25 (0.2%) N = 11,536 23 (0.2%) (N = 11,289) 2 (0.8%) N = 240 |
101 (1.4%) N = 6961 80 (1.2%) N = 6572 21 (5.5%) N = 380 |
126 (0.7%) N = 18,497 103 (0.6%) N = 17,861 23 (3.7%) N = 620 |
| Atony | 22(88%) | 65 (64.4%) | 87 (69%) |
| Birth Trauma | 0 | 30 (29.7%) | 30 (23.8%) |
| Retained Tissue | 2 (8%) | 3 (3%) | 5 (4%) |
| IV Fluid Infusion | 25 (100%) | 98 (97%) | 123 (97.6%) |
| Blood Transfusion | 3 (12%) | 1 (1%) | 4 (3.2%) |
| Given Uterotonic for PPH Management | 25 (100%) | 92 (91%) | 117 (92.9%) |
| Given Tranexamic Acid | 22 (88%) | 80 (79.2%) | 102 (81%) |
| Referred | 5 (20%) | 16 (15.8%) | 21 (16.7%) |
FRU: First referral unit, HSC: Heat-stable carbetocin, IV: Intravenous, PPH: Post Partum Hemorrhage, N: Number of cases wherein HSC was used.
The Person’s chi-square test was employed to assess the association between the use of HSC for PPH prevention and the type of health facility. The results indicate a significant association, with notably higher utilization of HSC for preventing PPH observed in FRU-level facilities compared to non-FRU facilities (p < 0.001). Additionally, the administration of HSC within one minute of delivery was also significantly more frequent in FRU facilities compared to non-FRU facilities (p < 0.001) [Table 3]. A total of 12 structured district-level review meetings (one per month) were conducted by the district authorities to oversee the consistent availability of the HSC drug and address any challenges, including adverse drug events, encountered during the implementation of the HSC model. Based on implementation learnings, the district authorities decided to scale-up the model in all 32 delivery points of Dewas district. Presently, discussions are ongoing for incorporation of the HSC model into the state program implementation plans, paving the way for a statewide scale-up.
| Indicator | FRU | non-FRU | Total | P Value |
|---|---|---|---|---|
| 1. Cases received HSC for PPH prevention | ||||
| HSC use | 11,289 (63.20%) | 6572 (36.80%) | 17,861 | P < 0.001 |
| HSC not Used | 247 (38.84%) | 389 (61.16%) | 636 | |
| Total | 11,536 (62.37%) | 6961 (37.63%) | 18,497 | |
| 2. Cases received HSC within one minute of delivery | ||||
| HSC administered within one min | 11,283 (63.93%) | 6365 (36.07%) | 17,648 | P < 0.001 |
| HSC administered after one min | 6 (2.82%) | 207 (97.18%) | 213 | |
| Total | 11,289 (63.20%) | 6572 (36.80%) | 17,861 | |
FRU: First referral unit
Discussion
Steps toward reducing the incidence and impact of PPH would significantly contribute to reducing maternal mortality and morbidity. Likewise, improving the overall quality of maternal healthcare to prevent and treat complications such as PPH is critical to attaining the Sustainable Development Goals Target 3.1.[15] Despite efforts to identify patients with increased risk for PPH, this life-threatening complication often occurs in women with no identifiable risk factors,[16] highlighting the importance of an effective uterotonic to be used in the third stage of labor. This study implemented an HSC model as part of the AMTSL strengthening initiative and reported no mortality amongst 18,497 cases managed and zero adverse events.
Guidelines are needed to provide optimal, evidence-based care to women experiencing PPH, especially if a newer drug is introduced into the health system. Capacity-building and supportive supervision led to a nearly universal use of uterotonics within one minute, proving an entry point for quality improvement of AMTSL practices. Even though there was an increase from 0.3% to 0.7% of reported PPH incidence, it could be because of better monitoring and the fact that all risk factors for PPH were not recorded in this study.
HSC must be used appropriately and not for labor induction or augmentation because even if the drug administration is discontinued immediately upon signs of fetal or maternal distress, the drug will remain active in the mother’s body for a prolonged period, raising the risk of maternal and fetal death. This implementation model demonstrated that it is possible to ensure the appropriate use of HSC in public health delivery sites in India as per global recommendations. The data shows that the current public health drug management system can ensure uninterrupted availability, and in only <3.5% of cases was the HSC not used for PPH prevention with minimal or no wastage.
While HSC can only be used immediately after birth for PPH prevention, oxytocin is still required for labor, delivery, and postpartum, as it has multiple uses. This necessitates vital drug procurement planning and will need information from large-scale multicentric studies to estimate the correct quantity of quality-assured drugs that need to be procured. The results showed that 2.9% of the admissions were deliveries occurring outside the facility, mainly in transit (88%), highlighting the importance of birth preparedness as an essential intervention for scale-up in countries.
Our study highlights a notable disparity in the utilization of HSC for preventing PPH between non-FRU-level facilities and FRU facilities. This difference may be attributed to insufficient knowledge and skill acquisition among non-FRU staff concerning the indications and contraindications of this newer drug, despite receiving a day-long training on HSC use prior to implementation of intervention. Given that HSC is a newer drug for the public health settings, the fear, and concerns among non-FRU staff regarding its potential misuse in contraindicated cases could possibly be a reason for its relatively lower uptake. Other factors such as the unavailability of specialist doctors and lack of adequate infrastructure and equipment to manage PPH-related complications at non-FRU-level facilities may also contribute to the lower adoption of HSC use. Similar obstacles have been identified as key barriers in previous studies on the uptake of misoprostol for PPH prevention in LMICs.[17]
Additionally, our study revealed higher compliance with HSC use recommendations, particularly the administration of HSC within one minute of delivery, in FRU facilities compared to non-FRU facilities. This underscores the need for establishing a structured mechanism for supportive supervision for health staff posted at non-FRU facilities to increase and ensure its optimal use.[18]
Delays in detecting or treating PPH can result in complications or death. A blood collection drape can help provide an objective, accurate, and early diagnosis of PPH, and delayed or inconsistent use of effective interventions could be addressed by a treatment bundle. The E-MOTIVE intervention resulted in a 60% lower risk of the primary outcome—a composite of severe PPH, laparotomy for PPH, or maternal death from PPH.[19] In this study, PPH was diagnosed by health providers based on visual estimations and may be a factor in the under-reporting of PPH, but no death was reported. A recent Cochrane review showed no evidence that quantitative estimation of blood loss reduces the need for uterotonic agents, blood transfusion, or volume expanders during PPH.[20]
The findings of our study confirm the appropriate use of HSC and its implementation feasibility in controlled public health settings. However, initial procurement of a newer uterotonic like HSC, maintaining its optimal as well as ensuring its uninterrupted supply, could be challenging while going to scale country-wide or while planning replication of this initially implemented model in similar settings of other LMICs.
While the cost-effectiveness of HSC has been well established, the principal hurdle lies in the initial higher procurement costs within the public health system. This requires high-level advocacy to convince the key decision-makers to procure HSC using their existing financial resources.
Carbetocin, an oxytocin analog, has been used for the prevention of PPH since 1997. The molecular differences enhance the stability of carbetocin, making it possible to formulate carbetocin into a highly stable product. Despite this, there is a potential risk of the public health system procuring nonstable variants, emphasizing the necessity for structured training on HSC targeting senior leadership and key decision-makers at both state and national levels. These trainings will be crucial to ensure that the procurement of HSC is reliable, effective, and adheres to stringent quality standards.
Strengths and Limitations of the Study
The main strength is that to the best of our knowledge, this is the first study from India on the use of HSC, which determined the feasibility and assessed the optimal use of HSC in the existing public health system. The results of this study can be used for policy decision-making and program purposes for using HSC as an alternative to oxytocin for the prevention of PPH in settings where oxytocin is of suspect quality.
Limitations of our study include data analysis on deliveries carried out at intervention facilities captured during the study time frame. We did not include women who gave birth while in transit (i.e., on their way) in the analysis due to insufficient documentation regarding the administration of uterotonics in such deliveries. Additionally, we did not collect additional data to ascertain the underlying reasons for relatively lower HSC utilization in non-FRU facilities. The potential factors might involve a higher incidence of contraindicated cases in non-FRU settings or issues related to knowledge, skills, fear, and apprehension among staff regarding the use of this newer drug for PPH prevention. Further research is necessary to delve into these reasons; however, such an investigation was beyond the scope of the current study.
Conclusion And Global Health Implications
The PPH continues to pose a significant clinical risk for maternal morbidity and mortality. There is a need for implementing global evidence-based recommendations, including using new uterotonics such as HSC, where the quality of oxytocin remains suspect. The results of our study suggest safe and appropriate utilization of HSC in line with the WHO recommendations within India’s public health system, making it a critical starting point for reinforcing AMTSL practices. The initial outcomes of implementing the HSC model in controlled settings are feasible for introduction and appropriate to use. It is crucial to comprehend how the existing supply chain and healthcare system adapt to and ensure the optimal utilization of HSC at the most remote levels while scaling up this model to other geographies.
Key Messages
Our study demonstrated the feasibility of introducing Heat-Stable Carbetocin (HSC) and its appropriate use for postpartum hemorrhage (PPH) prevention in the existing health system of Madhya Pradesh, India.
HSC is an alternative to oxytocin for preventing PPH in settings where oxytocin quality cannot be guaranteed. Capacity building of healthcare providers during Active Management of the Third Stage of Labor (AMTSL) will increase the effectiveness of care.
Health system adaptations are necessary to ensure the optimization of HSC utilization and sustainable scale-up.
Acknowledgments
We thank the National Health Mission of MP State and Dewas district Health departments, Facility in Charge, and Ferring Pharmaceuticals Safe Birth Project for their support and cooperation.
Compliance with Ethical Standards
Conflicts of Interest
All the authors declare no conflict of interest. USAID provided financial assistance for implementation through IPE Global as an implementation partner.
Financial Disclosure
Nothing to declare.
Funding/Support
This work was made possible by the support of the American people through the USAID under the terms of Cooperative Agreement Number 72038621CA00004. This paper’s contents represent the author’s views and do not reflect the views of the US Government.
Ethics Approval
This study was approved by Institutional Review Board (IRB) of the International Institute of Health Management Research (IIHMR), New Delhi. The approval number is IIHMR/D/SRB/2 and date is 20 July 2022.
Declaration of Patient Consent
Patient’s consent is not required as patient’s identity is not disclosed or compromised.
Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
This work was made possible by the support of the American people through the USAID and its implementing partner SAMVEG (Scaling up RMNCHA) project. This paper’s contents represent the author’s views and do not reflect those of the USAID or the U.S. Government.
Special Collection
This article is published as part of the special collection on prevention and treatment of postpartum hemorrhage in high-burden low- and middle-income countries: building cross-national evidence through implementation research.
REFERENCES
- Newborn and child health|UNICEF India. [Accessed 2024 Feb 19]. Available from: https://www.unicef.org/india/what-we-do/newborn-and-child-health
- India – Sample Registration System (SRS)-Special Bulletin on Maternal Mortality in India 2018-20. [Accessed 2024 Feb 19]. Available from: https://censusindia.gov.in/nada/index.php/catalog/44379
- Guidance Note on Prevention and Management of Postpartum Hemorrhage. Maternal Health Division, MoH & FW Government of India; 2015.
- WHO recommendations: uterotonics for the prevention of postpartum haemorrhage. [Accessed 2024 Feb 19]. Available from: https://www.who.int/publications-detail-redirect/9789241550420
- Updated: Uses of Medicines for Prevention and Treatment of Postpartum Hemorrhage and Other Obstetric Purposes|USAID Global Health Supply Chain Program. [Accessed 2024 Feb 19]. Available from: https://www.ghsupplychain.org/updated-uses-medicines-prevention-and-treatment-post-partum-hemorrhage-and-other-obstetric-purposes
- Quality of medicines for life-threatening pregnancy complications in low- and middle-income countries: A systematic review. PLoS One. 2020;15(7):e0236060.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Accessibility and potency of uterotonic drugs purchased by simulated clients in four districts in India. BMC Pregnancy and Childbirth. 2014;14(1):386.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Knowledge, attitudes, and practices related to uterotonic drugs during childbirth in Karnataka, India: A qualitative research study. PLoS One. 2013;8(4):e62801.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development and stability of a heat-stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle-income countries. J Pept Sci. 2018;24(6):e3082.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379(8):743-52.
- [CrossRef] [PubMed] [Google Scholar]
- How to use heat-stable carbetocin and tranexamic acid for the prevention and treatment of postpartum haemorrhage in low-resource settings. BMJ Glob Health. 2022;7(4):e008913. World Health Organization. WHO Model List of Essential Medicines - 22nd list, 2021
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WHO Model List of Essential Medicines – 23rd list, 2023. [Accessed 2024 Feb 19]. Available from: https://www.who.int/publications-detail-redirect/WHO-MHP-HPS-EML-2023.02
- The use and misuse of oxytocin: A study in rural Karnataka, India. BMC Proceedings.. 2012;6(Suppl 1):P12.
- [Google Scholar]
- Cost-effectiveness and budget impact of heat-stable carbetocin compared to oxytocin and misoprostol for the prevention of postpartum hemorrhage (PPH) in women giving birth in India. BMC Health Services Research. 2023;23(1):267.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- SDG Target 3.1|Maternal mortality: By 2030, reduce the global maternal mortality ratio to less than 70 per 100 000 live births. [Accessed 2024 Feb 19]. Available from: https://www.who.int/data/gho/data/themes/topics/sdg-target-3-1-maternal-mortality
- Practice bulletin no. 183: Postpartum hemorrhage. Obstet Gynecol. 2017;130(4):e168-86.
- [CrossRef] [PubMed] [Google Scholar]
- Barriers or gaps in implementation of misoprostol use for post-abortion care and post-partum hemorrhage prevention in developing countries: A systematic review. Reproductive Health. 2017;14(1):139.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Knowledge and barriers on correct use of modified guidelines for active management of third stage of labour: A cross sectional survey of nurse-midwives at three referral hospitals in Dares Salaam, Tanzania. Afr Health Sci. 2020;20(4):1908-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Randomized trial of early detection and treatment of postpartum hemorrhage. N Engl J Med. 2023;389(1):11-21.
- [CrossRef] [PubMed] [Google Scholar]
- Methods for blood loss estimation after vaginal birth. Cochrane Database Syst Rev. 2018;9(9):CD010980.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





