Translate this page into:
Overcoming Challenges in Conducting Clinical Trials in Minority Populations: Identifying and Testing What Works
✉Corresponding author email: reazuine@globalhealthprojects.org
Abstract
Participation in clinical trials is one of the greatest gifts that humanity can give to the fields of medicine and public health. Clinical trials are central in public health's mission to advance drug discovery. The enrollment and retention of participants, especially minority populations, is one of the most practical challenges of successfully implementing a clinical trial. In spite of these challenges, there are many reasons why a broader public participation in clinical trials is critical. The ability to generalize the scientific findings and the principles of equity, justice, and beneficence require an equitable distribution of the risks, benefits, and burdens of research for all classes and groups of people. A new methodology article published in this journal presents a promising framework for addressing minority recruitment and retention using what is known and using it innovatively to address a difficult problem facing clinical trials and public health. The innovative application of what is known in addressing a challenging problem, as this article presents, is worth the reading of all those interested in scientifically rigorous and ethically sound clinical trials that substantially comprise of diverse populations.
Keywords
Clinical trials
Minority enrollment challenges
Global health
Research Ethics
One of the greatest gifts that humanity can give to the fields of medicine and public health is participation in clinical trials. Since the emergence of drug discovery to curb human ailment and extend human life, clinical trials have led to the ultimate tools of medicine—be they drugs or medical devices. These tools are at the center of the practice and art of medicine and public health.[1] Apart from the gift of life, clinical trials have been at the center of scientific attempts to preserve life and bestow optimum quality of living.
Against this background, it is understandable why the number of clinical trials has increased over the last two decades. Between 2000 and 2015, the number of clinical trials registered in Clinical Trials.gov, a United States-government mandated database, increased from 5,600 to 185,000.[2] This accounts for almost 33 fold- increase from the inception of the database (Table 1 and Figure 1).
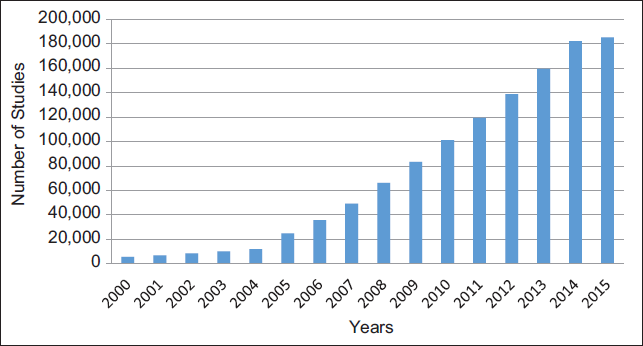
- Trends in the Number of Registered Studies in Clinical Trials.gov, 2000-2015 (as of March 3, 2015)
| Location | All Registered Studies | Studies Currently Recruiting | ||
|---|---|---|---|---|
| N | % | N | % | |
| Non-U.S. Only | 84,264 | 45 | 18,033 | 52 |
| U.S. Only | 72,296 | 39 | 14,428 | 42 |
| Both U.S. and Non-U.S | 11,071 | 6 | 2,061 | 6 |
| Not specified | 17,716 | 10 | N/A | N/A |
| Total | 185,347 | 34,522 | ||
Despite the centrality of clinical trials in public health and in the advancement of drug discovery, one of the greatest burdens for any clinical trial practitioner involved in the recruitment of participants into clinical trials is the enrollment of minority populations. This is not because minorities are averse to participating in clinical trials. No, it is, in part, because minorities have been at the receiving end of unethical, albeit atrocious, attempts to enroll participants in a clinical trial known in recent memory.[3] In the developed countries, these challenges are at the center of bioethical discourses in clinical research.
Although more pervasive among the minority populations, there are many reasons why the average members of the public, if they had a choice, would rather not participate in a clinical trial. These include not feeling that they can benefit from the ultimate products of the trial, low research literacy, and sometimes lack of congruency between the investigators and the populations researched. Of all these reasons, the most significant and underlying cause of immense public's, especially minority populations, trepidation to participate in clinical trials is a historical mistrust between researchers and potential clinical trials participants.
There are historical antecedents of unethical conduct of clinical trials that tend to justify the average member of public's reluctance, and sometimes outright refusal, to participate in clinical trials.[1,4] From the 1940s up to the late 1970s, a plethora of negative events occurred in the name of clinical trials that were both unethical and out rightly atrocious to say the least. A few of them are noteworthy. In 1947, Dr. Karl Brandt and 22 colleagues were convicted of conducting unethical human experiments on individuals incarcerated in the Nazi concentration camps. Brandt and his team had sterilized over 3.5 million German citizens.[4,5]
Between 1950s and 1972, children with mental disability at a State School in Willowbrook, Staten Island, New York, USA were intentionally infected with viral hepatitis in an unethical experimental quest to help discover vaccine.[4,5] In 1963, Dr. Chester Southam, another clinical researcher, injected live cancer cells to 22 elderly patients at the Jewish Chronic Disease Hospital in Brooklyn, New York, USA, with live cancer cells in an unethical bid to understand how the human body fights off malignant cells.[4,5] Perhaps, the most celebrated of these unethical studies was the infamous 1932 “Tuskegee Study of Untreated Syphilis in the Negro Male.” Unlike prior studies, in this study sponsored by a government public health agency, researchers informed black men that they were receiving treatment for Syphilis—which was not true.[3] Unethical studies are, by no means, limited to developed countries. In 1996, pharmaceutical giant Pfizer paid out thousands of dollars in compensation for conducting a Trovan study on children in Nigeria that raised fundamental issues around ethics and corruption in clinical trials.[4]
As complicated as they are, the issues surrounding Pfizer's incident in Nigeria highlight the deep vulnerability of the developing world at the hands of unethical clinical trials. The maternal and child health populations and people living with HIV/AIDS, majority of whom are in the developing world, are at the greatest point of vulnerability because of the need to cling to life. A number of reasons give rise to this increased vulnerability including lack of clinical research education, low public literacy on clinical trials, and weak and often corrupt legal systems that offer little or no protection for clinical trial participants who are unfairly treated while enrolled in clinical trials. The developing world is also an uncharted territory for western-based clinical trials practitioners. This is true given that 45% of ever- registered clinical trials in the world (as at March 3, 2015) were outside the United States.[1,2] We must be on the guard to protect the vulnerable in the developing world as an increasing number of clinical trials are moving to these climes.
Regardless of the aforementioned negative antecedents, there are many reasons why public participation in clinical trials must be encouraged. The ability to generalize the scientific findings and the principles of equity, justice, and beneficence require an equitable distribution of the risks, benefits, and burdens of research for all classes and groups of people.[5] How do we achieve equity in the face of the ongoing challenges? How do we ensure the 2000-2015 (as of March 3, 2015) recruitment of a diverse participant pool including minority populations in the face of a fractured history?
The methodology article by Salihu and his colleagues published in this journal sets the stage for this journey to the next steps.[6] The article presents the novel application of the socioecological model (SEM) as a framework for addressing recruitment and retention of minorities in a randomized clinical trial in southern part of the United States. First advanced by Sociologists at the Chicago School post-world-war and finessed by Bronfenbrenner between 1970s and the late 2000s, the SEM is not new.[7] What, however, is novel is the application of SEM in addressing a public health problem that is globally accepted to be challenging, albeit daunting. Salihu and his research team advance our knowledge using what is available, making it work, and showing that it works in a population that we so desperately need it to work.
The fact that, despite years of regulation and global roll-out of trainings and sometimes national apologies, public hesitancy to join clinical trials persist demonstrates that regulations may not have all the solutions. From recruitment, through retention, and follow-up, the challenges of enrolling participants in clinical trials persist. Public health must take the next step—and that is using what is available in our research and practice arsenals—to show what works and what does not work in rebuilding a broken trusting relationship between public health and the public. It is time for researchers themselves to look inwards and begin to identify evidence-based strategies for building trust with the public. This next step must include efforts to replicate those strategies that work so they can be tested and adopted across the world. The article by Salihu and colleagues is one of them.[6] Public health can improve by making use of the tools that we have in our hands today because the use of tools we have in hand can achieve dramatic improvements in outcomes.[8]
Public health agencies and pharmaceutical industry all have a part in this. It is high time that global health researchers and clinical trial experts applied this model in the hinterlands of Nigeria, in the mountains of Nepal, and in the inner cities of the Philippines. Salihu and colleagues have put the ball in the courts of public health and clinical trials practitioners worldwide, it is now our turn to engage and use the foundation they have laid to take this to the next step.
As a public health activity, the public must be at the center of clinical trials.[9] The fundamental hallmarks of engaging any population, especially minority populations, in clinical trials must include transparency, honesty, engagement, and investigator- population congruency. This is what Salihu and his team have done.[6] It is an example of what works in global health.[8] We must begin to test these promising models both in developed as well as in developing countries. This is what all of us must strive to do when we approach the next potential participant for our new clinical study.
The society has come a long way in making amends despite the historical atrocities that had plagued human enrollment in clinical trials. Our modern-day Institutional Review Boards, plethora of human subjects research trainings mandated by leading global health agencies are few of the attempts, over the years, to assuage public's fears to overcome mistrust and heal the black eye of clinical trials. The world must keep guard to ensure that these atrocities do not happen again. We need to make the public believe in the altruism of clinical trial practitioners.
Acknowledgment:
The authors acknowledge the mentorship and directions from instructors in several training opportunities including Introduction to Principles and Practice of Clinical Research at National Institutes of Health (NIH); Bioethics Intensive Course and fellowship at University of Maryland; and Translational Health Disparities Course (NIH). The views expressed here are those of the authors and do not necessarily reflect those of the Global Health and Education Projects or any of the authors’ affiliated institutions.
Conflict of Interest:
Authors declare no conflict of interest.
Funding:
The authors have no financial assistance to carry out the research.
References
- Designing Clinical Research. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
- [Google Scholar]
- ClinicalTrials.gov. Bethesda, MD: US Department of Health and Human Services. 2015 Available at https://clinicaltrials.gov/ct2/about-site/background
- [Google Scholar]
- The Tuskegee Timeline. Atlanta, GA: US Department of Health and Human Services Available at http://www.cdc.gov/tuskegee/timeline.htm
- [Google Scholar]
- Wikipedia. Abdullahi v Pfizer, Inc; Available at http://en.wikipedia.org/wiki/Abdullahi_v._Pfizer,_Inc. See also Unethical human experimentation in the United States. Available at http://en.wikipedia.org/wiki/Unethical_human_experimentation_in_the_ United_States
- [Google Scholar]
- Ethical and Regulatory Aspects of Clinical Research. Baltimore, MD: The Johns Hopkins University Press; 2003.
- [CrossRef]
- Socioecological model as a framework for overcoming barriers and challenges in randomized control trials in minority and underserved communities. International Journal of MCH and AIDS. 2015;3(1):85-95.
- [CrossRef] [Google Scholar]
- Ecological models of health behavior. In: Glanz K, Rimer BK, Lewis FM, eds. Health behavior and health education: Theory, research, and Practice. San Francisco, CA: John Wiley & Sons, Inc; 2002. 2002
- [Google Scholar]
- Ebola Virus Disease Epidemic: What can the World Learn and Not Learn from West Africa? International Journal of MCH and AIDS. 2014;3(1):1-6.
- [CrossRef] [Google Scholar]





