Translate this page into:
Detectable HIV-RNA Viral Load Among HIV-Infected Pregnant Women on Treatment in Northern Uganda
*Corresponding author email: napyoagnes@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background / Objectives:
Detectable HIV viral load among HIV-infected pregnant women remains a public health threat. We aimed to determine factors associated with detectable viral load among HIV-infected pregnant women in Lira, Northern Uganda.
Methods:
We conducted a cross-sectional survey among 420 HIV-infected pregnant women attending Lira Regional Referral Hospital using a structured questionnaire and combined it with viral load tests from Uganda National Health Laboratories. We conducted multivariable logistic regression while adjusting for confounders to determine the factors associated with detectable viral load and we report adjusted odds ratios and proportion of women with viral load less than 50 copies/ml and above 1000 copies, respectively.
Results:
The prevalence of detectable viral load (>50 copies/ml) was 30.7% (95%CI: 26.3% - 35.4%) and >1000 copies/ml was 8.1% (95% CI: 5.7% – 11.1%). Factors associated with detectable viral load were not belonging to the Lango ethnicity (adjusted odds ratio = 1.92, 95%CI: 1.05 – 3.90) and taking a second-line (protease inhibitor-based) regimen (adjusted odds ratio = 4.41, 95%CI: 1.13 – 17.22).
Conclusions and Global Health Implications:
HIV-infected pregnant women likely to have detectable viral load included those taking a protease inhibitor-based regimen and those who were not natives of Lira. We recommend intensified clinical and psychosocial monitoring for medication compliance among HIV-infected pregnant women that are likely to have a detectable viral load to significantly lower the risk of vertical transmission of HIV in Lira specifically those taking a protease inhibitor-based regimen and those who are non-natives to the study setting. Much as the third 90% of the global UNAIDS 90-90-90 target has been achieved, the national implementation of PMTCT guidelines should be tailored to its contextual needs.
Keywords
HIV
Women
Pregnancy
Pregnant women
Viral load
Viral suppression
PMTCT
Antiretroviral therapy
Uganda
1. Background and Introduction
Worldwide, there were 37.9 million people living with HIV (PLH) in 2018 and about 65% had access to antiretroviral therapy (ART) by June 2019. 1 Eastern and Southern Africa bear the biggest burden of the epidemic1 and Uganda is no exception.2 Women are disproportionately affected by HIV compared to males2 which translates into potential risk of HIV transmission from a mother to her offspring when getting pregnant. The World Health Organization as well as the Uganda national ART guidelines recommend combination ART for pregnant and breastfeeding HIV infected women regardless of their immune status,3,4 and the most common is a fixed drug combination taken once daily. In Uganda, more than 95% of HIV infected pregnant women received ART in 2017 to reduce the risk of HIV transmission to their offspring.2
One of the indicators used in the prevention of mother-to-child transmission of HIV-1 (PMTCT) programs is maternal plasma HIV-1 RNA viral load.5 Detectable or high maternal viral load increases risk of mother to child transmission of HIV.6 Detectable or high maternal viral load among HIV infected pregnant women has also been associated with various risk factors such as poor adherence to antiretroviral drugs7,8 and type of antiretroviral regimen being ingested.9–11 However, these risk factors vary across various study contexts therefore interventions targeted towards the virtual elimination of mother to child transmission of HIV in one context may not necessarily work for another – they may need to be context-specific.
Detectable viral load is a public health threat that can potentially translate into virologic failure and HIV drug resistance. We therefore set out to determine the factors associated with detectable viral load among HIV infected pregnant women in Lira, Northern Uganda. The objective of the study was to determine the factors associated with detectable viral load among HIV infected pregnant women in Lira, Northern Uganda.
2. Methods
2.1. Study Design
We conducted a cross-sectional study among 420 HIV positive pregnant women on ART between August 2018 and July 2019. We have followed the STROBE guidelines in drafting of this paper.12
2.2. Setting
Lira Regional Referral Hospital (LRRH) serves all 8 districts of the Lango sub region in Northern Uganda. It has an annual outpatient attendance of almost 100,000 patients, an annual antenatal care attendance of about 5,000 patients and conducts approximately 6,000 to 7,000 deliveries annually. Lira is one of the sentinel sites in Uganda with the highest antenatal HIV prevalence at 13.5%13 making it a suitable site for our study due to the availability of an accessible population of interest. HIV care and treatment at LRRH is entirely supported and offered freely by the Government of Uganda through the Ministry of Health. At the time of conducting the study, the Uganda national HIV care and treatment policy guidelines recommended that once an HIV-infected pregnant woman has been initiated on ART, viral load testing should be done six months after initiation of treatment and thereafter once every year.
2.3. Sample Size Estimation
We calculated a sample size for detecting a difference between two independent proportions using STATA version 14.0 (StataCorp; College Station, TX, USA). We assumed that 23% of HIV infected pregnant women had detectable viral load 14 and that 12% of HIV infected women that had detectable viral load were taking a protease inhibitor-based regimen.15 Two sample size calculations were done for the prevalence and factors associated respectively. After accounting for 11% non-response the final sample size was 420 (Figure 1). The sample size obtained was sufficient to cover the sample sizes required for estimating the prevalence as well as investigating the factors associated with detectable viral load.
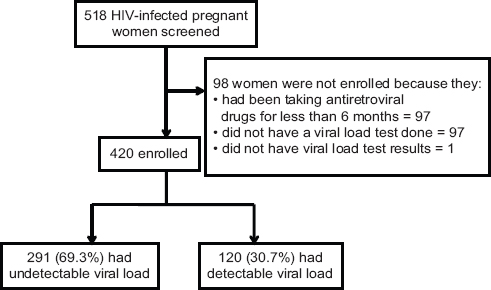
- Study flow chart
2.4. Participants
Study participants were identified, consented and recruited consecutively through the existing Ugandan program for HIV treatment, care and support for pregnant women at the PMTCT clinic. Participants were recruited onto the study as they came into the clinic until the estimated sample size was reached. Participants included in this study commenced their ART at different points in time and only those who had been on ART for at least six months or more, had viral load test done and results available were included in the analysis. The Ugandan ART treatment guidelines recommend viral load monitoring for HIV infected pregnant women who have taken ART for at least six months.4 Participants with a viral load count of 1,000 or more copies/ml were referred for intensive adherence counselling.
2.5. Study Variables
2.5.1. Dependent variable
Detectable viral load, the main outcome of the study, was defined as the presence of 50 or more copies of HIV-1 RNA per millilitre (ml) of blood plasma.3 Viral load counts below 50 copies/ml were categorized as ‘undetectable viral load’. This was done for comparability purposes. The presence of 1,000 or more copies/ml was called ‘viral non-suppression’. Blood was drawn from HIV-infected pregnant women who were due for viral load monitoring and the blood samples shipped under cold chain to the Uganda National Health Laboratories where the viral load tests were done following the recommended scheduled routine as per the consolidated guidelines for the prevention and treatment of HIV in Uganda.4 The Roche cobas 8800 system technology (Hoffmann-la roche Ltd, Basel, Switzerland) with a level of detection of 40 copies/ml was used for viral load testing. The recommended schedule for virologic monitoring among HIV-infected pregnant women was having the viral load monitored six months after initiation of ART and thereafter once every 12 months. Viral load results were then available after two to three weeks.
2.5.2. Independent variables and covariates
The independent variables and covariates included in the analysis were socio-demographic-related like age, education, marital status, employment status, religious affiliation, ethnicity and socioeconomic index. Reproductive-related covariates included were: parity, gestational age, birth control use and intention to have baby. HIV-related covariates included were: HIV status disclosure, ART regimen and duration of ART.
Ethnicity was categorized as ‘Lango ethnicity’ or ‘other’. Antiretroviral treatment was categorized as ‘efavirenz-based’, ‘nevirapine-based’ or ‘protease inhibitor-based’. The latter is commonly a second-line regimen.
We created a composite index of wealth (socio-economic status) using principle component analysis (PCA) because it is the most-suitable choice to use when calculating wealth indices from categorical variables.16 We used PCA on house ownership, availability of electricity in the house, source of drinking water and fuel used for cooking. Scores were obtained and categorized into four groups (quartiles) ranging from the poorest to the least poor.
The interviews were conducted in Lango (the main language spoken in the study setting) or in English by trained study staff. The interviews followed a structured questionnaire with questions on socio-demographic, reproductive-related and HIV-related information. The questions were translated into Lango and back translated into English to ensure accuracy and minimize interpretation bias.
2.6. Statistical Analysis
Data were entered by two independent data entrants using EpiData software (www.epidata.dk, version 4.4.3.1) and exported for analysis into Stata version 14.0 (StataCorp, College Station, Texas, USA.). Continuous data, if normally distributed, were summarized into means and standard deviations and if not, were summarized into medians with their corresponding interquartile ranges. Categorical variables were summarized into frequencies and percentages. The proportion of HIV-infected pregnant women with detectable viral load was estimated and its confidence limits calculated using the exact method. We used multivariable binary logistic regression to estimate the adjusted odds ratios (OR) of the independent variables on detectable viral load while controlling for other variables like age, education status, person to whom HIV status was disclosed and duration of taking ART. Initially, all these variables were included in the crude analyses. Variables with a p-value of <0.25 and those with biological or epidemiological plausibility were included in the final model.
2.7. Ethical Approval
Approval to conduct the study was granted by the Makerere University School of Medicine Research and Ethics Committee, the Uganda National Council for Science and Technology and the Norwegian Regional Committee for Medical and Health Research Ethics in the West. Administrative clearance was granted by the Lira district health officer and LRRH. Service providers / counsellors at the PMTCT clinic were introduced to the study and its procedures and were requested to identify, mobilize and link willing participants with the research team. Participants received verbal and written information detailing the purpose and process of the study. All participants provided written informed consent confirming their voluntary participation in the study. Those that declined participation were not penalised or denied standard health care. Confidentiality and privacy of all data collected was observed during the course of the study through restricted access.
3. RESULTS
3.1. Sociodemographic Characterisitcs
A total of 518 HIV infected pregnant women were screened for eligibility from the antenatal care clinic at LRRH (Figure 1) and 420 women were included in the analysis. The participants had a mean age of 30.0 (SD 5.2). Socio-demographic characteristics are presented in table 1a. More than half (54.1%) of the women were 30 years or more. A total of 197 (46.8%) had attained formal education for a duration of at least six years or more. The majority (95%) of the women were married (or cohabiting) and not formally employed (60.5%). They were predominantly Christian (96.2%) and Lango speaking (90.5%) (Tables 1a and 1b). A considerable proportion of these women had disclosed their HIV status (98.3%).
| Characteristics | Total (N=420) | Undetectable viral load <50 copies/ml (N=291) | Detectable viral load ≥50 copies/ml (N=129) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Socio-demographic | |||
| Age | |||
| ≤ 20 years | 17 (4.0) | 10 (3.4) | 7 (5.4) |
| 21 – 29 years | 176 (41.9) | 128 (44.0) | 48 (37.2) |
| ≥30 years | 227 (54.1) | 153 (52.6) | 74 (57.4) |
| Education | |||
| 0 – 6 years | 223 (53.1) | 155 (53.3) | 68 (52.7) |
| 7 – 10 years | 135 (32.1) | 90 (30.9) | 45 (34.9) |
| 11 – 13 years | 43 (10.3) | 31 (10.6) | 12 (9.3) |
| ≥14 years | 19 (4.5) | 15 (5.2) | 4 (3.1) |
| Marital status | |||
| Married | 399 (95.0) | 275 (94.5) | 124 (96.1) |
| Single | 21 (5.0) | 16 (5.5) | 5 (3.9) |
| Employment status | |||
| Employed | 166 (39.4) | 114 (39.2) | 52 (40.3) |
| Not employed | 254 (60.5) | 177 (60.8) | 77 (59.7) |
| Religious affiliation | |||
| Christian | 404 (96.2) | 280 (96.2) | 124 (96.1) |
| Moslem | 16 (3.8) | 11 (3.8) | 5 (3.9) |
| Ethnicity | |||
| Langi | 380 (90.5) | 268 (92.1) | 112 (86.8) |
| Other | 40 (9.5) | 23 (7.9) | 17 (13.2) |
| Socioeconomic index | |||
| Group 1 (poorest) | 105 (25.0) | 76 (26.1) | 29 (22.5) |
| Group 2 | 107 (25.5) | 73 (25.1) | 34 (26.4) |
| Group 3 | 145 (34.5) | 98 (33.7) | 47 (36.4) |
| Group 4 (least poor) | 63 (15.0) | 44 (15.1) | 19 (14.7) |
| Characteristics | Total (N=420) | Undetectable viral load <50 copies/ml (N=291) | Detectable Viral load ≥50 copies/ml (N=129) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Reproductive-related | |||
| Parity | |||
| 1 – 4 | 289 (68.8) | 203 (69.8) | 86 (66.7) |
| 5 – 9 | 131 (31.1) | 88 (30.2) | 43 (33.3) |
| Gestational age (in weeks) | |||
| 20 – 27 | 225 (53.6) | 158 (54.3) | 67 (51.9) |
| 28 – 35 | 138 (32.9) | 94 (32.3) | 44 (34.1) |
| ≥ 36 | 57 (13.5) | 39 (13.4) | 18 (14.0) |
| Accompanied to antenatal care by partner | |||
| Accompanied | 42 (10.2) | 31 (10.6) | 11 (8.5) |
| Not accompanied | 378 (89.8) | 260 (89.4) | 118 (91.5) |
| Use of birth control | |||
| Did not use | 198 (47.1) | 136 (46.7) | 62 (48.1) |
| Used 6 months prior to pregnancy | 222 (52.9) | 155 (53.3) | 67 (51.9) |
| Type of contraceptive used | |||
| None or “safe days” | 196 (46.7) | 133 (45.7) | 63 (48.8) |
| Effective contraception | 224 (53.3) | 158 (54.3) | 66 (51.2) |
| Intention to have baby | |||
| No | 182 (43.3) | 127 (43.6) | 55 (42.6) |
| Yes | 238 (56.7) | 164 (56.4) | 74 (57.4) |
| HIV-related | |||
| HIV status disclosure | |||
| Disclosed | 413 (98.3) | 287 (98.6) | 126 (97.7) |
| Not disclosed | 7 (1.7) | 4 (1.4) | 3 (2.3) |
| Fear about others’ opinion on HIV status | |||
| Had no fear | 199 (47.4) | 137 (47.1) | 63 (48.8) |
| Had fear | 221 (52.6) | 154 (52.9) | 66 (51.2) |
| Antiretroviral treatment | |||
| Efavirenz-based | 370 (88.1) | 263 (90.4) | 107 (83.0) |
| Nevirapine-based | 39 (9.3) | 24 (8.2) | 15 (11.6) |
| Protease inhibitor-based | 11 (2.6) | 4 (1.4) | 7 (5.4) |
| Duration of antiretroviral treatment | |||
| 6 – 36 months | 216 (51.4) | 160 (55.0) | 56 (43.4) |
| 37 – 119 months | 180 (42.9) | 121 (41.6) | 59 (45.7) |
| ≥ 120 months | 24 (5.7) | 10 (3.4) | 14 (10.9) |
3.2. Outcome Variable
The majority of the participants had undetectable viral load (Table 2). The prevalence of detectable viral load (>50 copies/ml) was 30.7% (95% CI: 26.3% - 35.4%). Of those with detectable viral load, the majority (n=82, 19.5% 95% CI: 15.8% – 23.6%) had viral load between 50 and 400 cps/ml. The proportion of women with a viral load ≥1000 cps/ml was 8.1% (95% CI: 5.7% – 11.1%).
| Viral load count (copies/ml) | Frequency (n=420) | Percentage (%) |
| <50 | 291 | 69.3 |
| 50 to 400 | 82 | 19.5 |
| 401 to 999 | 13 | 3.1 |
| ≥ 1000 | 34 | 8.1 |
3.3. Covariates
Not belonging to the Lango ethnicity was associated with having detectable viral load among HIV infected pregnant women. Women belonging to other groups of ethnicity were almost twice as likely to have detectable viral load as their Lango counterparts (Table 3). Women who were on second-line treatment regimen were four times likely to have detectable viral load as those who had been taking a first-line efavirenz-based regimen.
| Variables | Unadjusted OR | Adjusted OR |
|---|---|---|
| (95% CI) | (95% CI) | |
| Age | ||
| ≤ 20 years | 1.45 (0.53 – 3.95) | 0.85 (0.29 – 2.53) |
| 21 – 29 years | 0.78 (0.50 – 1.19) | 0.73 (0.46 – 1.16) |
| ≥ 30 years | 1 | 1 |
| Education status | ||
| 0 – 6 years | 1 | 1 |
| 7 – 10 years | 1.14 (0.72 – 1.8) | 1.21 (0.75 – 1.96) |
| 11 – 13 years | 0.88 (0.43 – 1.82) | 0.85 (0.40 – 1.81) |
| Tertiary | 0.61 (0.19 – 1.90) | 0.52 (0.16 – 1.76) |
| Ethnicity | ||
| Langi | 1 | 1 |
| Other | 1.77 (0.91 – 3.44) | 1.92 (1.05 – 3.90) |
| Person disclosed to | ||
| Spouse | 1 | 1 |
| Sibling | 0.67 (0.36 – 1.22) | 0.68 (0.36 – 1.26) |
| Other | 1.2 (0.54 – 2.70) | 1.32 (0.57 – 3.05) |
| Antiretroviral treatment | ||
| Efavirenz-based | 1 | 1 |
| Nevirapine-based | 1.54 (0.77 – 3.04) | 1.25 (0.58 – 2.71) |
| Protease inhibitor-based | 4.3 (1.23 – 14.99) | 4.41 (1.13 – 17.22) |
| Duration of antiretroviral treatment | ||
| 6 – 36 months | 0.72 (0.46 – 1.11) | 0.66 (0.41 – 1.06) |
| 37 – 119 months | 1 | 1 |
| ≥ 120 months | 2.87 (1.20 – 6.85) | 2.08 (0.77 – 5.53) |
4. Discussion
4.1. Discussion
One third of our study’s participants had detectable viral load which is higher than that documented in other studies using the same cut-off of 50 copies/ml. Studies from South Africa11,14,17 and Malawi18 documented lower prevalence of detectable viral load among HIV infected pregnant women ranging from 10% to 23%. Much as various studies have demonstrated the association between detectable viral load and poor or non-adherence to ART,7,19–22 we cannot speculate non-adherence as a plausible explanation for the high prevalence of undetectable viral load since we did not measured non-adherence at this point in time.
The cut-off used for detectable viral load will also determine its prevalence. The lower the cut-off, the higher the prevalence is likely to be. A study in Malawi 23 reported a similar prevalence of detectable viral load like our study, while researchers from Rwanda24 reported a higher prevalence than ours. These studies, however, used a lower cut-off. In our study, the prevalence of virologic non-suppression (>1000 cps/ml) was 8%. Studies in Uganda22 and Malawi25 reported a similar prevalence of virologic non-suppression among HIV infected pregnant women. The low prevalence of virological non-suppression among women presenting for antenatal care in Lira can be used as an argument for the success of the universal treatment program in maintaining viral suppression and of the progress towards the last 90% of the UNAIDS 90-90-90 target.26 Much as we have achieved the desired target for viral suppression today, the cut-off of 1,000 copies/mL used in Uganda is too high. This puts the focus on HIV-infected pregnant women with higher virologic profiles and less emphasis on those with lower but detectable HIV-1 RNA, and yet it is those with detectable viral loads that are likely to translate into virologic non-suppression.
It is rather surprising that our study found that women who belong to other groups of ethnicity other than the Lango ethnicity (that is predominant in the study setting) had a higher likelihood of having a detectable viral load. A study in Benin27 found the opposite, that the predominant ethnicity of the study setting was more likely to have a detectable viral load. More qualitative studies are needed in our study context to understand reasons for this finding.
We also found that taking a second-line protease inhibitor-based regimen increased the odds of having detectable viral load, which is quite a common finding. Studies in Uganda9 and the United States of America28 found that protease inhibitor-based regimens were associated with lower probability of viral suppression than first-line efavirenz-based and nevirapine-based regimens among HIV infected pregnant women. However, there was no difference in the rate of viral suppression among women using protease inhibitor-based regimens and those using efavirenz-based or nevirapine-based regimens in the postpartum period.9,29,30. This finding also be can be explained further by the concept of reverse causality. HIV-infected women will be switched to a second-line ART regimen because of treatment failure of the first-line regimens which is mainly due to non-adherence.31,32 It is probable that the underlying challenges of adherence that forced these women onto second-line regimens may be aggravated during the use of second-line ART regimens. Non-adherence may be influenced by a number of factors and these may include side effects to antiretroviral drugs, pill burden in the context of pregnancy and increased psychosocial stressors related to pregnancy or care giving.31
4.2. Limitations
Our study had some limitations. This study was done in a hospital setting; therefore, the findings may only be generalizable to the study context or those similar to it. We did not report any adherence rates at this point in time because this is a baseline study of a cohort of HIV-infected pregnant women for whom adherence to ART will be measured as follow-up is being done. However, a strength of our study is that it is the first to describe virologic profiles among HIV-infected pregnant women in Northern Uganda and also demonstrates that in fact disparities in viral suppression do exist across various groups of ethnicity in this study setting.
5. Conclusion and Global Health Implications
HIV-infected pregnant women likely to have detectable viral load included those taking a protease inhibitor-based regimen and those who were not natives of Lira. Having a detectable viral load during pregnancy increases the risk of MTCT of HIV. We recommend intensified clinical and psychosocial monitoring of medication compliance among HIV-infected pregnant women that are likely to have detectable viral load to significantly lower the risk of vertical transmission of HIV in Lira specifically those taking a protease inhibitor-based regimens and those who are non-natives to the study setting. Much as the third 90% of the global UNAIDS 90-90-90 target33 has been achieved in the study setting, the national implementation of PMTCT guidelines should be inclusive of HIV-infected pregnant women that are most at-risk of having detectable viral load in this study context and those similar to it.
Acknowledgements:
We are grateful to Lira regional referral hospital, the study participants and the research assistants for their contribution to this survey.
Compliance with Ethical Standards
Conflicts of Interest: The authors declare no conflict of interest.
Funding Disclosure: The study was funded by the Norwegian Programme for Capacity Development in Higher Education and Research for Development (NORHED) by the Norwegian Agency for Development Cooperation (Norad), Norway through the Survival Pluss Project at Makerere University (no. UGA-13-0030).
Ethics Approval: The study received ethical approval from the following issuing/approving bodies: Uganda National Council for Science and Technology; Norwegian Regional Committee for Medical and Health Research Ethics in the West; and Makerere University College of Health Sciences School of Medicine Research and Ethics (SOMREC) committee.
References
- 2019. Global HIV Statistics. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- 2016. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection:Recommendations for a Public Health Approach. https://www.who.int/hiv/pub/arv/arv-2016/en/
- 2016. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. https://aidsfree.usaid.gov/sites/default/files/uganda_hiv_gl_2016.pdf
- Factors associated with lack of viral suppression at delivery among highly active antiretroviral therapy-naive women with HIV a cohort study. Ann Intern Med. 2015;162(2):90-99. doi:10.7326/M13-2005
- [Google Scholar]
- HIV-exposed uninfected infants in Zimbabwe :insights into health outcomes in the pre-antiretroviral therapy era. Front Immunol. 2016;7(1):190. doi:10.3389/fimmu.2016.00190
- [Google Scholar]
- Multivariate analysis of covariates of adherence among HIV - positive mothers with low viral suppression. AIDS Res Ther. 2018;15(1):9. doi:10.1186/s12981-018-0197-8
- [Google Scholar]
- Virologic and immunologic failure , drug resistance and mortality during the first 24 months postpartum among HIV-infected women initiated on antiretroviral therapy for life in the Mitra plus Study , Dar es Salaam , Tanzania. BMC Infect Dis. 2015;15(1):175. doi:10.1186/s12879-015-0914-z
- [Google Scholar]
- Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS. 2017;29(7):825-830. doi:10.1097/QAD.0000000000000619
- [Google Scholar]
- HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95%. J Acquir Immune Defic Syndr. 2007;45(1):4-8. doi:10.1097/QAI.0b013e318050d8c2
- [Google Scholar]
- Factors associated with time to achieve an undetectable HIV RNA viral load after start of antiretroviral treatment in HIV-1-infected pregnant women. J Virus Erad. 2017;3(1):34-39.
- [Google Scholar]
- Strengthening the reporting of observational studies in epidemiology (STROBE):Explanation and elaboration. Int J Surg. 2014;12(1):1500-1524. doi:10.1016/j.ijsu.2014.07.014
- [Google Scholar]
- Same-day antiretroviral therapy ( ART ) initiation in pregnancy is not associated with viral suppression or engagement in care :A cohort study. J Int AIDS Soc. 2018;21(1):e25133. doi:10.1002/jia2.25133
- [Google Scholar]
- Risk factors for detectable HIV-1 RNA at delivery among women receiving highly active antiretroviral therapy in the women and infants transmission study. J Acquir Immune Defic Syndr. 2010;54(1):27-34. doi:10.1097/QAI.0b013e3181caea89
- [Google Scholar]
- Constructing socio-economic status indices:How to use principal components analysis. Health Policy Plan. 2006;21(6):459-468. doi:10.1093/heapol/czl029
- [Google Scholar]
- HIV viraemia during pregnancy in women receiving preconception antiretroviral therapy in KwaDukuza , KwaZulu-Natal. South Afr J HIV Med. 2019;20(1):a847. doi:10.4102/sajhivmed.v20i1.847
- [Google Scholar]
- Viro-immunological response and emergence of resistance in HIV-infected women receiving combination antiretroviral regimens for the prevention of mother-to-child transmission in Malawi. J Antimicrob Chemother. 2018;69(1):749-752. doi:10.1093/jac/dkt408
- [Google Scholar]
- Self-reported antiretroviral therapy adherence and viral load in criminal justice-involved populations. BMC Infect Dis. 2019;19(1):913-923. doi:10.1186/s12879-019-4443-z
- [Google Scholar]
- Relationship between viral load and behavioral measures of adherence to antiretroviral therapy in children living with HIV in Latin America. Brazilian J Infect Dis. 2015;19(3):263-271. doi:10.1016/j.physbeh.2017.03.040
- [Google Scholar]
- Adherence to antiretroviral therapy during and after pregnancy in low-, middle and high income countries:A systematic review and meta-analysis. AIDS. 2016;26(16):2039-2052. doi:10.1097/QAD.0b013e328359590f.Adherence
- [Google Scholar]
- Factors associated with virological non- suppression among HIV-positive patients on antiretroviral therapy in Uganda , August 2014 –July 2015. BMC Infect Dis. 2017;17(1):326. doi:10.1186/s12879-017-2428-3
- [Google Scholar]
- Optimizing prevention of HIV mother to child transmission:Duration of antiretroviral therapy and viral suppression at delivery among pregnant Malawian women. PLoS One. 2018;13(4):e0195033. doi:10.1371/journal.pone.0195033
- [Google Scholar]
- Detectable Viral Load in Late Pregnancy among Women in the Rwanda Option B +PMTCT Program :Enrollment Results from the Kabeho Study. PLoS One. 2016;11(12):e0168671. doi:10.1371/journal.pone.0168671
- [Google Scholar]
- Prevalence of antiretroviral therapy treatment failure among HIV-infected pregnant women at first antenatal care:PMTCT option b+in Malawi. PLoS One. 2018;13(12):e0209052. doi:10.1371/journal.pone.0209052
- [Google Scholar]
- 2014. 90-90-90 An Ambitious Target to Help End the AIDS Epidemic. https://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf
- Predictive factors of plasma HIV suppression during pregnancy:A prospective cohort study in Benin. PLoS One. 2013;8(3):e59446. doi:10.1371/journal.pone.0059446
- [Google Scholar]
- Time to viral load suppression in antiretroviral- naive and -experienced HIV-infected pregnant women on highly active antiretroviral therapy :implications for pregnant women presenting late in gestation. BJOG. 2013;120(1):1534-1547. doi:10.1111/1471-0528.12226
- [Google Scholar]
- Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49(5):485-491.
- [Google Scholar]
- Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda:A qualitative study. BMC Pregnancy Childbirth. 2017;17(1):94-102. doi:10.1186/s12884-017-1276-x
- [Google Scholar]
- Predictors of nonadherence to single-dose nevirapine therapy for the prevention of mother-to-child HIV transmission. J Acquir Immune Defic Syndr. 2007;41(1):114-118.
- [Google Scholar]
- 2018. UNAIDS Data 2018. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf





