Translate this page into:
Factors Impacting Vaccine Uptake during Pregnancy: A Retrospective Analysis
* Corresponding author email: deepa.0424@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 4.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background and Objective:
Vaccine uptake rates during pregnancy remain below target goals due to a convergence of factors. In particular, women of lower socioeconomic means and racial minorities typically have reduced rates of vaccine acceptance. This study aims to identify additional factors contributing to vaccine acceptance within a sample population of women receiving prenatal care in Houston, Texas, United States of America.
Methods:
We performed a retrospective cross-sectional analysis of 11,500 pregnant women covered by Medicaid or ChipPerinate who received prenatal care during 2013-2021, assessing influenza (flu) and combined Tetanus, Diphtheria, Acellular Pertussis (TDAP) vaccine acceptance in the patient population. We examined temporal trends in flu and TDAP vaccination rates using Joinpoint regression analyses and evaluated the factors associated with single or concomitant vaccine acceptance during the study period and during the COVID-19 pandemic using adjusted log-binomial regression models.
Results:
In our population, 54% of patients received flu vaccination, and 76.1% received TDAP. TDAP rates increased from 2013-2015 but have shown an overall decline since then, as with the flu vaccine. Earlier entry to prenatal care (Prevalence Ratio [PR] 6.32; Confidence Interval [CI] 3.28-12.24) and pregnancy comorbidity such as gestational diabetes (PR 1.32; CI 0.82-2.19) were positively associated with uptake. In contrast, the NH-Black race was negatively associated with vaccine acceptance (PR 0.51 CI; 0.25-0.99). Otherwise, age and history of pre-pregnancy comorbidities were not significant predictors.
Conclusion and Global Health Implications:
Within demographic groups identified as at-risk for vaccine refusal, modifying factors further impact vaccine hesitancy. Identifying these elements will guide targeted patient efforts to promote vaccine uptake, both for routine prenatal recommendations and for COVID vaccination.
Keywords
Vaccination in Pregnancy
TDAP
Flu
Patient Communication
Vaccine Hesitancy in Pregnancy
COVID-19
1. Introduction
Vaccination in pregnancy remains an area for improvement. The two consistently recommended vaccinations in pregnancy are seasonal influenza (flu) vaccine and the combined Tetanus, Diphtheria, Acellular Pertussis (TDAP) vaccine.1 Despite efforts to increase rates of vaccination, average rates of vaccination across US populations for either vaccine were approximately 55%.2
As of early November 2017, flu vaccination coverage among pregnant women in the United States before and during pregnancy was 35.6%.2 In the same year, TDAP vaccination coverage during pregnancy among women who had a live birth was 50.4%, similar to TDAP vaccination coverage in 2016 (48.8%).3 The reasons for low vaccination rates vary and have included noted associations with certain demographic populations. In particular, pregnant women of Hispanic or Black race/ethnicity and low socioeconomic status have been noted to be less likely to accept vaccination.4
Vaccine recommendations in pregnancy are provided to help promote the safety and health of a mother and fetus throughout pregnancy and into the early stages of a child’s life. For some infectious diseases, such as flu, tetanus, and pertussis, increasing the concentration of maternal antibodies through vaccinating pregnant women is the only option of offering passive protection to the child after birth.5 Pregnant women are encouraged to obtain flu vaccines during any trimester of their pregnancy.6 It is also recommended that pregnant women receive the TDAP vaccine during weeks 27-36 of gestation, ideally at the earlier part of the third trimester, to help ensure adequate vertical transfer of maternal antibodies to the fetus before birth. This can help protect the child during the early months of life until it can also be vaccinated with TDAP at 2 months of age.7 Rarely, other vaccinations may be indicated in pregnancy; however, TDAP and flu have remained the primary recommended vaccinations prior to the COVID-19 pandemic.
Racial and ethnic disparities have repeatedly been documented in vaccine uptake. In an April 2020 US Centers for Disease Control and Prevention’s (CDC) online survey, over 1,800 pregnant women were surveyed and asked specifically about whether they received the flu and/or TDAP vaccine. Overall, 61.2% of respondents received the flu vaccine, 56.6% received the TDAP vaccine, and 40.3% of respondents reported having received both vaccines. However, overall vaccination rates were lowest among Black (23%) and Hispanic respondents (25.4%).8
Studies have revealed a number of factors associated with increased prenatal vaccine uptake, including the availability of educational materials, comprehensive discussions on rationale and safety, and a strong recommendation from the patient’s physician.9,10 Other noted positive associations with increased vaccine uptake are the availability of vaccines on-site, private insurance, and use of assisted reproductive technologies.11
A 2016 multinational literature review found that factors associated with lower vaccine uptake were younger maternal age, smoking, not married, Hispanic or Black race/ethnicity, having less than a university degree, living below the poverty line, lack of health insurance, history of preterm delivery, and not having an obstetrical care provider.12 Barriers to vaccination that were identified by pregnant women included concerns about vaccine safety (especially for the fetus and newborn), concerns about vaccine effectiveness, lack of vaccine knowledge, mistrust of vaccines, perceived disease severity, perceived risk of disease, lack of recommendation to receive vaccines, societal factors (social norms, family influence, and religion), fear of needles, access to vaccination services, and having to pay for the vaccine and administration costs.12
There have not been many studies examining factors associated with vaccine hesitancy in racial/ethnic minority pregnant populations. Our study aims to fill this lacuna in the literature. This study seeks to delve more deeply to identify additional factors which may be associated with vaccine acceptance or refusal amongst pregnant women of low socioeconomic status and predominantly minority race/ethnicity. By identifying those women who are most likely to decline vaccination, targeted efforts can be made to address the unique barriers and concerns in this subgroup. The target population in this study represents a traditionally medically underserved group and, unfortunately, also represents the population most at risk in the current COVID-19 pandemic. A clearer understanding of women most likely to decline vaccines during pregnancy will further inform patient outreach initiatives and COVID vaccination efforts.
2. Methods
2.1. Study Design
A retrospective analysis of all pregnant women who received prenatal care at a patient-centered medical home clinic in Houston, Texas, USA, from January 2013 - March 2021 was conducted with a review of an existing prenatal care database. The prenatal clinic provides prenatal care for women covered by Medicaid or Chip Perinate, two of the Texas state government’s health care insurance plans that provide no-cost prenatal care based on income criteria. All patients included in the study met income eligibility criteria for Medicaid or CHIP coverage in pregnancy.13 The study was approved by the Baylor College of Medicine’s Institutional Review Board.
2.2. Study Variables
The study considered several exposure variables – age at first prenatal visit divided into 5-year increments, along with the age extremes of pregnancy. Race/ethnicity was classified as non-Hispanic (NH) White, NH-Black Hispanic, and NH-Others. Gestational age at the time of first visit was also analyzed and divided into 1st trimester (< 13 weeks); 2nd trimester (13-28 weeks); and early (29-34 weeks); and late 3rd trimester (35-40 weeks). Gestational age at entry to prenatal care was used as a proxy for length of relationship with providers and for dosage or magnitude of exposure to the intervention, which was vaccine acceptance. Women with first-time or repeat pregnancies visiting the center were identified.
Consideration was also given to pregnancy comorbidities as covariates, including pre-pregnancy and gestational diabetes, hypertension, and a history of prior preterm delivery. These particular conditions were considered higher than normal pregnancy risk conditions and are noted within the prenatal database. These conditions typically necessitated more regular communication with the clinical provider team. For the study outcome, we created a composite variable, which was flu and TDAP concordant acceptance; women who accepted both flu and TDAP vaccines were marked as ‘1’ and the ones who denied either or both vaccines were marked as ‘0.’ We also analyzed the acceptance of each vaccine type independently.
2.3. Statistical Analysis
All statistical tests of hypotheses were two-tailed with a type-1 error rate set at 5%. We examined the patient demographic, comorbid, and vaccine acceptance characteristics among our study population and the results were represented using frequency distributions. We assessed flu and combined TDAP vaccine acceptance in the patient population stratified by patients’ age, race/ethnicity and their repeat pregnancy status (i.e., first time or repeat pregnancy at the center).
We examined temporal trends in flu and TDAP vaccination rates using Joinpoint regression analyses. Joinpoint regression model started by assuming zero joinpoints (straight line) to fit the rates of the outcome over time. Then recursively and iteratively, singular joinpoints were added and the model fit was evaluated. The final model with optimal number of joinpoints which achieved statistical significance was selected to obtain the average annual percentage change (AAPC) and 95% confidence interval (CI) for the change in the rates of outcomes over the study duration. Finally, we evaluated the factors associated with single or concomitant vaccine acceptance during the study period and during the COVID-19 pandemic (i.e., 2020-2021) using adjusted log-binomial regression models. All statistical analyses were performed using R (version 3·5·1),14 RStudio (Version 1·1·423)15, and temporal trends analyses were performed using Joinpoint Regression Program 4.6.0.0 (National Cancer Institute, Bethesda, MD).16
3. Results
3.1. Sociodemographic Information
Our study included data from 11,500 pregnant patients in the analysis during the study period from January 2013 – March 2021. Demographics of the patients in this study indicate that the majority were Hispanic (67.3%, n=7,745) or NH-Black (23.6%, n=2,712) and that most were between 20-24 (26%) or 25-29 (24.5%) years of age at the time of first prenatal visit. An overwhelming majority (83.6%, n=8,111) of patients were repeat pregnancies, and most patients (58.5%) attended their first prenatal visit between gestational weeks 13-28. Regarding comorbidities, 2.2% (n=252) of patients reported having pre-gestational diabetes while 6.8% (n=781) reported having gestational diabetes. Additionally, 19.8% (n=2,278) of patients were classified as high-risk patients, 17.0% (n=1,951) reported a history of preeclampsia, and 1.8% (n=206) reported a history of preterm births. Of the surveyed patients, 54% (n=6,206) received the flu vaccine, and 76.1% (8,757) received the TDAP vaccine. Together, 50.6% (n=5,815) of patients received both the flu and TDAP vaccine, 5.7% (n=660) received the flu vaccine only, 26.6% (n=3,057) received the TDAP vaccine only, and 17.1% (n=1,971) received neither the flu nor TDAP vaccine (Table 1).
| N=11,503 | %=100 | |
|---|---|---|
| Age of the patient at the time of first prenatal visit | ||
| <20 years | 867 | 7.5% |
| 20-24 years | 2,992 | 26% |
| 25-29 years | 2,813 | 24.5% |
| 30-34 years | 1,989 | 17.3% |
| 35-39 years | 1,018 | 8.8% |
| 40+ years | 267 | 2.3% |
| Missing | 1,557 | 13.5% |
| Race/ethnicity | ||
| Non-Hispanic White | 529 | 4.6% |
| Non-Hispanic Black | 2,712 | 23.6% |
| Hispanic | 7,745 | 67.3% |
| Non-Hispanic Others | 517 | 4.5% |
| Method of delivery | ||
| Vaginal | 4,260 | 37% |
| Cesarean | 2,591 | 22.5% |
| Active pregnancies | 561 | 4.9% |
| Other (miscarriage/ectopic/missing) | 4,091 | 35.6% |
| Newborn gender | ||
| Female | 4,699 | 40.8% |
| Male | 4,938 | 42.9% |
| Missing | 1,866 | 16.2% |
| Gestational Age at first visit | ||
| <13 weeks | 3,477 | 30.2% |
| 13-28 weeks | 6,726 | 58.5% |
| 29-34 weeks | 951 | 8.3% |
| 35-40 weeks | 332 | 2.9% |
| Missing | 17 | 0.1% |
| Newborn living status | ||
| Alive | 9,110 | 79.2% |
| Dead | 101 | 0.9% |
| Missing | 2,292 | 19.9% |
| Women with Repeat pregnancies | ||
| No | 8,111 | 83.6% |
| Yes | 1,587 | 16.4% |
| Pre-gestational Diabetes | ||
| No | 11,251 | 97.8% |
| Yes | 252 | 2.2% |
| Gestational Diabetes | ||
| No | 10,722 | 93.2% |
| Yes | 781 | 6.8% |
| History of Preeclampsia | ||
| No | 9,552 | 83% |
| Yes | 1,951 | 17% |
| History of Preterm birth | ||
| No | 11,297 | 98.2% |
| Yes | 206 | 1.8% |
| High risk patient | ||
| No | 2,278 | 19.8% |
| Yes | 9,225 | 80.2% |
| Flu vaccination status | ||
| Declined | 5,028 | 43.7% |
| Given | 6,206 | 54% |
| Not eligible | 269 | 2.3% |
| TDAP vaccination status | ||
| Declined | 2,746 | 23.9% |
| Given | 8,757 | 76.1% |
| Combined vaccination status | ||
| Not given Flu or TDAP vaccination | 1,971 | 17.1% |
| Given Flu vaccination but not TDAP vaccine | 660 | 5.7% |
| Given TDAP vaccination but not Flu vaccine | 3,057 | 26.6% |
| Given both Flu and TDAP vaccination | 5,815 | 50.6% |
3.2. Vaccine Acceptance and Age
Figure 1 shows the vaccine acceptance by patients’ age. Among those who accepted only flu but not TDAP, the highest rate was among those <20 years (5%), and the lowest was among those 40+ years (1.9%). The highest rate of TDAP vaccination alone was in the 30-34 years group (29.6%), whereas the lowest rate was in the 40+ years group (25.1%). Among those who accepted both flu and TDAP vaccinations, the highest rate of 57.3% was in the 40+ years age group, whereas the lowest was in the 35-39 group (54.2%).
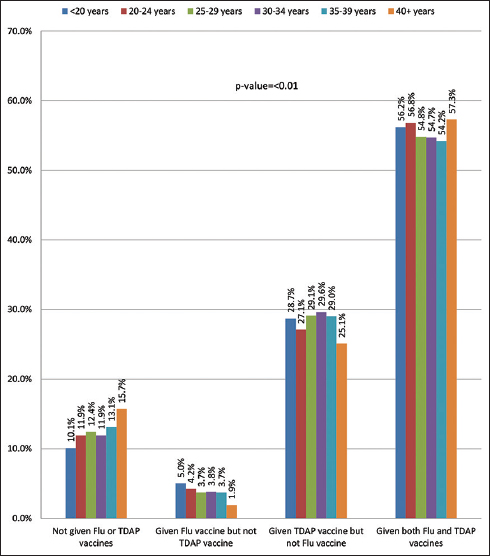
- Flu and TDAP vaccine acceptance by patient’s age
3.3. Vaccine Acceptance by Race/Ethnicity
Figure 2 depicts the differing rates of TDAP and flu vaccine acceptance among NH-White, NH-Black, Hispanic, and NH-Other patient populations. Among patients who reported not having received either vaccine, NH-Black patients were the most represented at 23.4%, followed by NH-Other (19.7%), NH-White (18.1%), and Hispanic patients (14.7%). The smallest subset of patients were those who received only the flu vaccine, and, of this group, NH-White patients were the most represented at 6.4%, and NH-Black patients (5.2%) were the least represented. The patients who only received the TDAP vaccine were the second largest group in the study and were mostly comprised of NH-White patients (30.6%). Patients who received both vaccines were the largest group, and, of these patients, Hispanic patients were the most represented at 54.2%, whereas NH-Black patients (41.3%) were the least represented.

- Flu and TDAP vaccine acceptance by patient’s race/ethnicity
3.4. Vaccine Acceptance Rate and Repeat Pregnancy Status
The vaccine acceptance for flu alone, TDAP alone, or flu+TDAP was almost the same among those who were first-time patients and those who were repeat patients at the center. Overall, we observed that there were no statistically significant differences in vaccine acceptance by the repeat pregnancy status at the center (Figure 3).
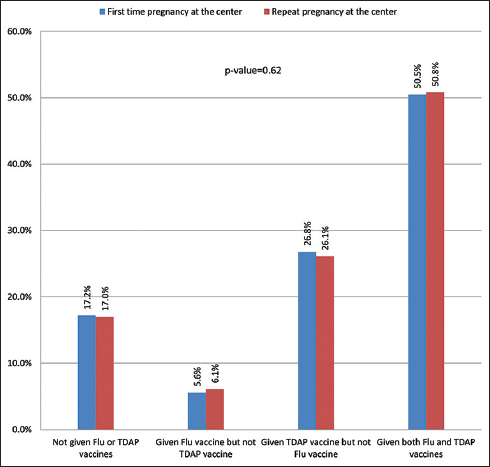
- Flu and TDAP vaccine acceptance by patient’s repeat pregnancy status at the center
3.5. Trends in Flu and TDAP Vaccine Acceptance
Figure 4 shows the temporal trends of flu and TDAP vaccine acceptance rates during the study period from 2013 to 2021. During this period, there was a 2.7% decrease in the flu vaccine acceptance rate per year (95% CI, -5.1 to -0.2). Regarding the TDAP vaccine, there was an initial 11.7% increase in acceptance rate per year from 2013 to 2015 (95% CI, 3.1 to 21.1), but from 2015 to 2021, there was a 4.4% decrease in acceptance rate per year (95% CI, -5.7 to -3.1). Overall, there was a 0.6% decrease in the TDAP vaccine acceptance rate per year; however, this average annual percentage change did not achieve statistical significance due to the drastic shift in trend.

- Temporal trends analysis of flu and TDAP vaccine acceptance over the study period- 2013-2021
- APC-Annual percentage change, AAPC-Average annual percentage change
3.6. Factors Associated with Flu, TDAP, and Both Flu+TDAP Vaccine Acceptance
Table 2 shows the factors associated with flu, TDAP, concomitant flu, and TDAP vaccine acceptance in our study population from adjusted log-binomial regression models. When compared with those who are less than 20 years of age, we observed a lower prevalence of flu plus TDAP vaccine acceptance in women aged 30-39 (PR= 0.71, 95% CI= 0.54-0.93) and 40+ age group (PR=0.55, 95% CI=0.35-0.87). For both vaccines, NH-Black women were less likely than NH-White women to receive the vaccinations. (PR= 0.8 for flu vaccine, PR= 0.64 for TDAP, and PR= 0.63 for concomitant vaccine). Receipt of flu vaccine had a 45% increased likelihood in Hispanic women, as compared to NH-White women (95% CI =1.19-1.78). The results demonstrated an inverse dose-response relationship between the initiation of prenatal care and acceptance of all vaccines, with the highest likelihood being in those who initiated prenatal care in the first trimester and the lowest in those who started in the last trimester. Diabetes, whether pre-gestational or diagnosed during pregnancy, was also associated with increased vaccine acceptance.
| Flu vaccine acceptance | TDAP vaccine acceptance | Flu and TDAP vaccine acceptance | |
|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Age of the patient at the time of first prenatal visit | |||
| <20 years | Reference | Reference | Reference |
| 20-29 years | 0.95 (0.81-1.11) | 0.86 (0.69-1.06) | 0.75 (0.57-0.96) |
| 30-39 years | 0.87 (0.74-1.03) | 0.86 (0.68-1.08) | 0.71 (0.54-0.93)* |
| 40+ years | 0.9 (0.67-1.22) | 0.84 (0.55-1.28) | 0.55 (0.35-0.87)* |
| Race/ethnicity | |||
| Non-Hispanic White | Reference | Reference | Reference |
| Non-Hispanic Black | 0.80 (0.64-0.99)* | 0.64 (0.48-0.86)* | 0.63 (0.45-0.85)* |
| Hispanic | 1.45 (1.19-1.78)* | 1.12 (0.84-1.48) | 1.30 (0.95-1.76) |
| Non-Hispanic Others | 1.36 (1.02-1.80)* | 1.04 (0.70-1.54) | 1.05 (0.69-1.61) |
| Method of delivery | |||
| Vaginal | Reference | Reference | Reference |
| Cesarean | 0.92 (0.82-1.02) | 0.68 (0.58-0.79)* | 0.69 (0.58-0.82)* |
| Other (miscarriage/ectopic/missing) | 0.82 (0.74-0.91)* | 0.42 (0.37-0.48)* | 0.45 (0.39-0.53)* |
| Newborn gender | |||
| Female | Reference | Reference | Reference |
| Male | 1.04 (0.95-1.13) | 1.00 (0.89-1.12) | 0.97 (0.85-1.10) |
| Gestational Age at first visit | |||
| 35-40 weeks | Reference | Reference | Reference |
| <13 weeks | 4.54 (3.44-6.03)* | 7.69 (5.82-10.17)* | 8.74 (6.53-11.68)* |
| 13-28 weeks | 3.13 (2.39-4.13)* | 7.42 (5.69-9.68)* | 7.70 (5.86-10.09)* |
| 29-34 weeks | 1.80 (1.33-2.44)* | 3.11 (2.30-4.20)* | 2.91 (2.14-3.95)* |
| Pre-gestational Diabetes | |||
| No | Reference | Reference | Reference |
| Yes | 1.39 (1.03-1.89)* | 1.13 (0.76-1.75)* | 1.3 (0.82-2.19) |
| Gestational Diabetes | |||
| No | Reference | Reference | Reference |
| Yes | 1.02 (0.87-1.21) | 1.26 (0.99-1.62) | 1.32 (1.01-1.76)* |
| History of Preeclampsia | |||
| No | Reference | Reference | Reference |
| Yes | 0.92 (0.82-1.03) | 0.91 (0.78-1.06) | 0.89 (0.75-1.05) |
| History of Preterm birth | |||
| No | Reference | Reference | Reference |
| Yes | 0.76 (0.55-1.04) | 0.95 (0.63-1.49) | 0.90 (0.58-1.46) |
| High risk patient | |||
| No | Reference | Reference | Reference |
| Yes | 1.00 (0.89-1.12) | 1.01 (0.86-1.18) | 1.01 (0.85-1.21) |
3.7. Factors Associated with Flu, TDAP, and Both Flu+TDAP Vaccine Acceptance during COVID-19 Period
Table 3 delves into the factors associated with flu, TDAP, and concomitant vaccination during the current COVID-19 pandemic, focusing on 2020- early 2021 period. In this time period also, the NH-Black race was associated with a lower rate of acceptance for flu vaccine and alone, as well as concomitant acceptance (PR=0.53; 95% CI= 0.31-0.91; PR= 0.51; 95% CI= 0.25-0.99, respectively). Again, a significant inverse dose relationship was demonstrated between the timing of entry to prenatal care and vaccine acceptance.
| Flu vaccine acceptance | TDAP vaccine acceptance | Flu and TDAP vaccine acceptance | |
|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Age of the patient at the time of first prenatal visit | |||
| <20 years | Reference | Reference | Reference |
| 20-29 years | 0.97 (0.66-1.41) | 0.81 (0.51-1.26) | 0.76 (0.45-1.24) |
| 30-39 years | 0.93 (0.62-1.38) | 0.85 (0.52-1.35) | 0.82 (0.47-1.38) |
| 40+ years | 0.79 (0.4-1.57) | 0.77 (0.36-1.74) | 0.53 (0.23-1.24) |
| Race/ethnicity | |||
| Non-Hispanic White | Reference | Reference | Reference |
| Non-Hispanic Black | 0.53 (0.31-0.91)* | 0.66 (0.35-1.18) | 0.51 (0.25-0.99)* |
| Hispanic | 1.28 (0.76-2.15) | 1.39 (0.75-2.45) | 1.29 (0.63-2.42) |
| Non-Hispanic Others | 1.28 (0.62-2.66) | 1.21 (0.53-2.77) | 1.00 (0.4-2.49) |
| Method of delivery | |||
| Vaginal | Reference | Reference | Reference |
| Cesarean | 1.32 (0.23-7.46) | 1.76 (0.23-9.44) | 2.48 (0.33-13.35) |
| Other (miscarriage/ectopic/missing) | 1.48 (0.26-8.25) | 1.97 (0.26-10.47) | 2.90 (0.39-15.4) |
| Newborn gender | |||
| Female | Reference | Reference | Reference |
| Male | 0.96 (0.79-1.15) | 0.78 (0.63-0.97)* | 0.85 (0.67-1.07) |
| Gestational Age at first visit | |||
| 35-40 weeks | Reference | Reference | Reference |
| <13 weeks | 4.17 (2.14-8.55)* | 5.54 (2.92-10.7)* | 6.32 (3.28-12.24)* |
| 13-28 weeks | 3.36 (1.76-6.78)* | 5.21 (2.82-9.79)* | 5.65 (3.04-10.52)* |
| 29-34 weeks | 1.58 (0.77-3.37) | 1.95 (0.99-3.89) | 1.86 (0.94-3.69) |
| Pre-gestational Diabetes | |||
| No | Reference | Reference | Reference |
| Yes | 0.87 (0.47-1.60) | 1.34 (0.65-3.05) | 1.13 (0.53-2.73) |
| Gestational Diabetes | |||
| No | Reference | Reference | Reference |
| Yes | 1.13 (0.78-1.64) | 1.35 (0.87-2.17) | 1.27 (0.79-2.13) |
| History of Preeclampsia | |||
| No | Reference | Reference | Reference |
| Yes | 0.85 (0.68-1.08) | 0.94 (0.72-1.24) | 0.91 (0.68-1.22) |
| History of Preterm birth | |||
| No | Reference | Reference | Reference |
| Yes | 0.29 (0.04-1.15) | 0.63 (0.18-2.24) | 0.48 (0.14-1.70) |
| High risk patient | |||
| No | Reference | Reference | Reference |
| Yes | 1.11 (0.88-1.41) | 1.00 (0.76-1.32) | 0.97 (0.71-1.31) |
4. Discussion
Our study sought to identify the demographic factors most associated with vaccine uptake in pregnancy, as well as those negatively associated with vaccine acceptance. With this information, the intent is to develop targeted and specific strategies to address vaccine barriers among this group. Past studies have demonstrated that straightforward measures such as physician education programs or the distribution of informative posters can increase rates of vaccination.17 A combined effort of patient education aimed specifically towards demographic groups at risk for non-vaccination seems a practical direction to increase vaccine uptake.
While the study identified patient demographic characteristics negatively and positively associated with vaccination, our results also revealed an important association with the timing of initiated prenatal care. This area is far less studied; however, some studies have suggested the impact of a positive association with antenatal care, such as one Ivory Coast study, which noted a positive association between antenatal visits and uptake of tetanus vaccine and malaria prevention treatment.18 This finding was of particular interest in our study. One may argue that earlier initiation of care may simply represent heightened patient vigilance and patient medical compliance overall. However, we interpret these findings differently.
Our study, which used gestational age at the time of initiation of prenatal care as a proxy measure for length of relationship with providers, found a significant association between earlier onset of antenatal care and vaccine acceptance. This suggests that cultivating a longer relationship between providers and patients increases the likelihood of vaccine acceptance. This finding suggests that patients with late entry to prenatal care, and hence limited interaction with the provider team, should represent an additional demographic group that requires special attention and counseling regarding vaccination. For patients who enter into prenatal care at a later gestational age, particular attention should be given to foster trust and familiarity with the provider team. As will be discussed further, a variety of models have been discussed regarding the development and fostering of the patient-provider relationship. Our findings suggest that developing such a critical relationship may be foundational to vaccine uptake.
As noted above, in addition to the length of the provider-patient relationship, our study identified and highlighted key demographic characters associated with vaccine decision-making. The rate of vaccine acceptance in pregnant populations is influenced by a myriad of factors, including ethnicity,19,20 single motherhood,21,22 living below the poverty line,23 and younger age.19,20,23 These results begged the question of how to best counsel and discuss vaccination with these most vulnerable groups. Again, while many frameworks exist, our work suggests that a patient-centered approach may be most effective, with the goal of understanding vaccine hesitance, providing patient-appropriate education, and outlining clear recommendations.24 Direct communication between the patient and health care provider is critical to removing certain barriers impeding vaccine acceptance and educating patients on the benefits of vaccinations. For example, lack of knowledge of both TDAP and influenza negatively impacts maternal vaccination rates,25–28 but education from a health care provider on the importance of vaccinations during pregnancy leads to more positive attitudes and overall higher rates of vaccinations.29
Health care providers play an important role in dispelling common misperceptions about flu and TDAP vaccinations and ameliorating patient concerns about vaccine safety,30,31 side-effects,32,33 and potential harm to the child such as birth defects28,34 or miscarriage.35,36 Importantly, studies have found that patients are open to these conversations but are often less impacted when the information and recommendation are delivered as a pamphlet instead of direct contact with the health care provider,37,38 impacting their decision on whether to get vaccinated or not.
Ultimately, the result of direct patient-provider communication is evidenced by improved vaccine acceptance rates; research into this field has consistently shown a strong correlation between patient vaccine acceptance, especially flu and TDAP, in response to a direct recommendation from a health care provider. For example, a meta-analysis of the literature demonstrated that pregnant women who received a health care recommendation were 10 times more likely to get the TDAP vaccine and 12 times more likely to get the flu vaccine compared to patients who did not receive a recommendation.39 Taken together, this data serves to highlight the critical role that patient-centered communication plays in maternal vaccine rates.
4.1. Limitations
As the study was a retrospective database review, we could not conduct discussion groups with patients to better understand barriers to vaccination. In addition, some data points were incomplete within the database. Another limitation of our study is that most patients in our database were of Hispanic and NH-Black descent, with minimal comparison to other racial minority groups, such as American Indians and Alaskan Natives, Asians, and Pacific Islanders. Additionally, our study did not investigate other factors mentioned in outside articles, such as single motherhood or living below the poverty line. For future direction, more studies should be conducted that include an analysis of these factors.
4.2. Recommendations
Our study demonstrated that older NH-Black women with later initiation of prenatal care were the least likely to accept flu and TDAP vaccinations. We posit that the most effective next step would be a patient-centered communication approach to address the concerns of these women least likely to accept vaccination. Certainly, the same principles are applicable to all patients.
For patients most likely to decline vaccination, it is particularly critical to begin developing earnest patient-provider rapport immediately, given the demonstrated impact of trusted provider recommendations. When faced with a limited opportunity to cultivate partnership, a patient relationship-centered communication model may be a very effective tool.
Formal training in this communication model may, in turn, positively impact vaccine uptake.
5. Conclusion and Global Health Implications
Our study demonstrates several key points. Patients with certain demographic characteristics, including older age and Black race, along with patients who have a limited length of relationship with providers, are least likely to accept vaccination. The best approach to encourage necessary vaccination among these patients is through trustful and respectful communication. While the framework for communication may demonstrate inequities in different populations, the foundational approach of patient and relationship-centered communication is a critical first step in improving vaccine uptake.
Acknowledgments:
Texas Children’s Health Plan Centers for Children and Women Academy of Communication in Healthcare.
Compliance with Ethical Standards
Conflicts of Interest: The authors declare no competing interests.
Financial Disclosure: Nothing to declare.
Funding/Support: This research was supported by grant number 1 D34HP31024-01-00 from the Health Resources and Services Administration (HRSA) for the project titled Baylor College of Medicine (BCM) Center of Excellence in Health Equity, Training & Research. Ethics Approval: The study was approved by the Baylor College of Medicine’s Institutional Review Board.
Disclaimer: None.
References
- Update on immunization and pregnancy:tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130(3):e153-e157. doi:10.1097/AOG.0000000000002301
- [Google Scholar]
- Flu and TDAP Vaccination Coverage Among Pregnant Women –United States, April 2021. CDC. Page Last Reviewed October 7, 2021 https: //www.cdc.gov/flu/fluvaxview/pregnant-women-apr2021.htm
- [Google Scholar]
- Pregnant Women and TDAP Vaccination, Internet Panel Survey, United States, April 2017. CDC. Page Last Reviewed May 8, 2018 https: //www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/tdap-report-2017.html
- [Google Scholar]
- A Comparison of influenza &TDAP vaccination rates by characteristics of child-bearing-aged women. Health Sciences Research Commons. Published 2017 https: //hsrc.himmelfarb.gwu.edu/son_dnp/6
- [Google Scholar]
- Vaccination during pregnancy:current and possible future recommendations. Eur J Pediatr. 2020;179(2):235-42. doi:10.1007/s00431-019-03563-w
- [Google Scholar]
- Influenza (Flu) Vaccine and Pregnancy. CDC. Page Last Reviewed December 12, 2019 https: //www.cdc.gov/vaccines/pregnancy/hcp-toolkit/flu-vaccine-pregnancy.html
- [Google Scholar]
- Get the Whooping Cough Vaccine During Each Pregnancy. CDC. Page Last Reviewed June 10, 2021 https: //www.cdc.gov/pertussis/pregnant/mom/get-vaccinated.html
- [Google Scholar]
- Influenza and TDAP Vaccination Coverage Among Pregnant Women —United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1391-1397. doi:10.15585/mmwr.mm6939a2
- [Google Scholar]
- Knowledge and attitiudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine. 2015;33(41):5445-51. doi:10.1016/j.vaccine.2015.08.028
- [Google Scholar]
- Patient attitudes toward influenza and tetanus, diphtheria and acellular pertussis vaccination in pregnancy. Vaccine. 2018;36(30):4548-54. doi:10.1016/j.vaccine.2018.05
- [Google Scholar]
- Factors associated with TDAP vaccination receipt during pregnancy:a cross-sectional study. Public Health. 2020;179:38-44. doi:10.1016/j.puhe.2019.10.001
- [Google Scholar]
- Improving rates of maternal immunization:challenges and opportunities. Hum Vaccin Immunother. 2016;12(4):857-65. doi:10.1080/21645515.2015.1101524
- [Google Scholar]
- Texas Health and Human Services. https: //www.hhs.texas.gov/services/health/medicaid-chip/medicaid-chip-programs-services/programs-children-families/childrens-medicaid-chip
- 2017. R:A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; https: //www.R-project.org/
- Version 4.9.0.1- February 2022. Statistical Methodology and Applications Branch, Surveillance Research Program. National Cancer Institute. https: //surveillance.cancer.gov/joinpoint/
- [Google Scholar]
- Influenza vaccination during pregnancy and factors for lacking compliance with current CDC guidelines. J Matern Fetal Neonatal Med. 2011;24(3):402-6. doi:10.3109/14767058.2010.497882
- [Google Scholar]
- Antenatal visits are positively associated with uptake of tetanus toxoid and intermittent preventive treatment in pregnancy in Ivory Coast. BMC Public Health. 2019;19(1):1467. doi:10.1186/s12889-019-7847-1
- [Google Scholar]
- Influenza Vaccination Coverage Among Pregnant Women —United States, 2016–17 Influenza Season. MMWR Morb Mortal Wkly Rep. 2017;66(38):1016-1022. doi:10.15585/mmwr.mm6638a2
- [Google Scholar]
- Uptake of influenza vaccine in pregnant women during the 2009 H1N1 influenza pandemic. Am J Obstet Gynecol. 2011;204(6):S112-S5. doi:10.1016/j.ajog.2011.01.007
- [Google Scholar]
- The National Vaccine Advisory Committee:reducing patient and provider barriers to maternal immunizations:approved by the National Vaccine Advisory Committee on June 11, 2014. Public Health Rep. 2015;130(1):10-42. doi:10.1177/003335491513000104
- [Google Scholar]
- Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. CMAJ. 2014;186(4):E157-64. doi:10.1503/cmaj.130499
- [Google Scholar]
- Vaccination patterns in pregnant women during the 2009 H1N1 influenza pandemic:a population-based study in Ontario, Canada. Can J Public Health. 2012;103(5):e353-e8. doi:10.1007/BF03404440
- [Google Scholar]
- The importance of the patient voice in vaccination and vaccine safety—are we listening? Clin Microbiol Infect. 2016;22 Suppl 5:S146-S153. doi:10.1016/j.cmi.2016.09.027
- [Google Scholar]
- Influenza vaccination during pregnancy:coverage rates and influencing factors in two urban districts in Sydney. Vaccine. 2013;31(47):5557-64. doi:10.1016/j.vaccine.2013.08.081
- [Google Scholar]
- Uptake of influenza vaccination in pregnancy amongst Australian Aboriginal and Torres Strait Islander women:a mixed-methods pilot study. BMC Res Notes. 2015;8:169. doi:10.1186/s13104-015-1147-3
- [Google Scholar]
- Novel pandemic A (H1N1) influenza vaccination among pregnant women:motivators and barriers. Am J Obstet Gynecol. 2011;204(6, Suppl 1):S116-S23. doi:10.1016/j.ajog.2011.02.036
- [Google Scholar]
- Barriers to influenza vaccination among pregnant women. Vaccine. 2013;31(27):2874-8. doi:10.1016/j.vaccine.2013.04.031
- [Google Scholar]
- A cross-sectional study of maternity care providers'and women's knowledge, attitudes, and behaviours towards influenza vaccination during pregnancy. J Obstet Gynaecol Can. 2008;30(5):404-10. doi:10.1016/s1701-2163(16)32825-0
- [Google Scholar]
- Exploring pregnant women's views on influenza vaccination and educational text messages. Prev Med. 2011;52(1):75-7. doi:10.1016/j.ypmed.2010.10.009
- [Google Scholar]
- [Reasons why pregnant women did not vaccinate against influenza A H1N1] Cienc Saude Colet. 2013;18(6):1745-52.
- [Google Scholar]
- Pandemics and vaccines:perceptions, reactions, and lessons learned from hard-to-reach Latinos and the H1N1 campaign. J Health Care Poor Underserved. 2012;23(3):1106-22. doi:10.1353/hpu.2012.0086
- [Google Scholar]
- Vaccination against seasonal flu in Switzerland:the indecision of pregnant women encouraged by healthcare professionals. Rev Epidemiol Sante Publique. 2012;60(6):447-53. doi:10.1016/j.respe.2012.03.008
- [Google Scholar]
- Determinants of 2009 A/H1N1 influenza vaccination among pregnant women in Hong Kong. Matern Child Health J. 2013;17(1):23-32. doi:10.1007/s10995-011-0943-1
- [Google Scholar]
- Understanding barriers and predictors of maternal immunization:Identifying gaps through an exploratory literature review. Vaccine. 2018;36(49):7445-55. doi:10.1016/j.vaccine.2018.10.046
- [Google Scholar]
- H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102(6):e33-40. doi:10.2105/AJPH.2011.300606
- [Google Scholar]
- Vaccination against pertussis and influenza in pregnancy:a qualitative study of barriers and facilitators. Public Health. 2018;162:111-117. doi:10.1016/j.puhe.2018.05.025
- [Google Scholar]
- Reasons for use and non-use of the pertussis vaccine during pregnancy:an interview study. J Prim Health Care. 2016;8(4):344-50. doi:10.1071/HC15049
- [Google Scholar]
- Factors that influence vaccination decision-making among pregnant women:A systematic review and meta-analysis. PLoS One. 2020;15(7):e0234827. doi:10.1371/journal.pone.0234827
- [Google Scholar]





