Translate this page into:
Implementing WHO's Differentiated Service Delivery Model for Pregnant and Breastfeeding Women and Infants Living with HIV: Insights from Kenyan Healthcare Providers

*Corresponding author: John Humphrey, Department of Medicine, Indiana University School of Medicine, Indianapolis, Indiana, USA humphrjm@iu.edu
-
Received: ,
Accepted: ,
How to cite this article: Humphrey J, Carlucci JG, Wanjama EK, Naanyu V, Muli L, Alera JM, et al. Implementing WHO's differentiated service delivery model for pregnant and breastfeeding women and infants living with HIV: Insights from Kenyan healthcare providers. Int J MCH AIDS. 2025;14:e004. doi: 10.25259/IJMA_43_2024.
Abstract
Background and Objective:
Differentiated service delivery (DSD) is a strategy endorsed by the World Health Organization that simplifies and adapts human immunodeficiency (HIV) services to meet the needs of people living with HIV (PLHIV) while reducing unnecessary health system burdens. DSD for PLHIV has been widely adopted in sub-Saharan Africa, but DSD for women and infants enrolled in prevention of mother-to-child HIV transmission (PMTCT) services is lacking.
Methods:
We conducted in-depth interviews with healthcare providers (i.e., clinicians, nurses, and mentor mothers) in antenatal and postnatal clinics at two facilities affiliated with the Academic Model Providing Access to Healthcare (AMPATH) in Kenya to explore perspectives on the adaptation of DSD for PMTCT. Providers were recruited in person at each facility. Interview guides focused on their views on DSD implementation for PMTCT, characteristics of stable and unstable PMTCT clients, and strategies to improve PMTCT services. We used inductive coding with illustrative quotes to highlight emerging themes.
Results:
12 PMTCT providers (6 antenatal, 6 postnatal; 4 clinicians, 4 nurses, and 4 mentor mothers) were enrolled; 10 (83%) were female, with a median age of 40 years, and a median of 7 years of PMTCT experience. Providers held positive views about the potential benefits of DSD for PMTCT but expressed concern about reducing service intensity during pregnancy/breastfeeding. Providers also suggested specific criteria defining stable PMTCT clients beyond those used for non-pregnant PLHIV, such as having no pregnancy complications, psychosocial or socioeconomic barriers, or breastfed infants.
Conclusion and Global Health Implications:
Filling the gap in DSD guidance for this population will require adaptations to the DSD model that are responsive to providers’ concerns and the unique aspects of the pregnancy-postpartum service continuum, which may vary across settings based on contextual and client-level factors. Such nuanced guidance will need to remain clear and simple to implement to ensure implementation fidelity at scale.
Keywords
Africa
Health Services
HIV
Public Health Practice
Vertical Infectious Disease Transmission
Viremia
INTRODUCTION
Differentiated service delivery (DSD) is an evidence-based strategy endorsed by the World Health Organization (WHO) that adapts HIV services to meet the needs and preferences of people living with HIV (PLHIV).[1,2,3] By simplifying services for “clinically stable” clients (e.g., individuals retained in care and virally suppressed), DSD enables intensification of services for “unstable clients” (e.g., at risk of care disengagement or viremia).[2,3] This patient-centered approach diverges from standard-of-care HIV service delivery in many settings in which all clients receive similar services. DSD is premised on two principles: (1) client-centered care that acknowledges clients’ barriers and empowers them to manage their disease with the support of the health system; (2) improving health system efficiency by shifting resources to those most in need while supporting clients already established on antiretroviral therapy (ART).[4] Evidence suggests DSD models for PLHIV yield retention and viral suppression rates similar to or superior to the standard of care and improve program efficiency.[5,6,7,8] The strain on health systems during the COVID-19 pandemic has accelerated the implementation of DSD in sub-Saharan Africa.[9] Consequently, DSD models for non-pregnant/postpartum PLHIV have expanded regionally.[4,10,11,12] Yet, despite the scale-up of DSD, its implementation for pregnant and postpartum women living with HIV (WLHIV) and their infants is lacking, and there are limited data to guide its adaptation to the 1.4 million WLHIV who become pregnant each year globally.[13,14,15,16]
Multiple studies have found that pregnant and postpartum WLHIV are at increased risk of attrition and HIV viremia, leading to excess risk of vertical transmission, horizontal transmission to male partners, and maternal and infant morbidity and mortality.[17] These epidemiologic trends suggest compelling opportunities to implement DSD for the prevention of mother-to-child HIV transmission (PMTCT) population. The global expansion of HIV testing and early antiretroviral treatment (ART) initiation has also yielded an increasing proportion of WLHIV clinically stable on ART at enrollment in PMTCT services. In western Kenya, for example, 80% of WLHIV enrolling in PMTCT services had initiated ART prior to pregnancy, and 88% of these women were virally suppressed.[18] These women, some of whom were enrolled in DSD prior to pregnancy, are required to attend more frequent clinic appointments during pregnancy and throughout breastfeeding, lasting up to 18–24 months postpartum, regardless of risk factors for unfavorable outcomes. Although well-intended and designed to enhance clinical support for PMTCT, these frequent visits burden clients,[19] and there is little evidence that they improve HIV-related outcomes for WLHIV who are already clinically stable. Given emerging data demonstrating very low vertical transmission among pregnant and breastfeeding mothers who maintain undetectable viral loads, DSD could play a critical role in simplifying care for these clients while preserving good outcomes.[19,20,21] The massive global scale-up of ART for PTMCT has strained care systems; donor HIV funding is declining, and there is growing consensus that a “one-size-fits-all” approach to HIV service delivery will not be sufficient to ensure sustainable ART access for all PLHIV, eliminate new HIV infections in children, and end the AIDS epidemic.[11,12,22,23,24] DSD could help address these challenges by improving the efficiency, quality, and cost-effectiveness of PMTCT services for this population.[25]
Kenya has one of the largest HIV burdens in the world, with an HIV prevalence of 6.1% among reproductive-aged women.[26] Kenya’s HIV treatment guidelines reflect WHO's criteria to determine whether a person is established on ART and eligible for DSD.[27,28,29] These criteria are: receiving ART for at least 6 months; no current illness; good understanding of lifelong adherence; and suppressed viral load within the past 6 months. WHO and Kenya guidelines recommend that pregnant and postpartum WLHIV be included in DSD, though no guidance is given as to how to adapt the model to this unique population.[27,28] Consequently, we have observed disparate implementation practices among facilities in western Kenya, with some facilities maintaining an undifferentiated model and some allowing for unstructured appointment spacing after 6–12 months post-delivery. Ideally, such DSD implementation should be guided by the lived experiences and perspectives of its end users. The objective of our study was to understand providers’ perspectives regarding the implementation of DSD for PMTCT in Kenya.
METHODS
Setting
This study was conducted at public health facilities affiliated with the Academic Model Providing Access to Healthcare (AMPATH). AMPATH supports the care and treatment of HIV and other chronic diseases across a network of >300 Ministry of Health facilities in 17 counties in western Kenya based on national guidelines.[27] Through PEPFAR support, AMPATH provides HIV care and treatment to >170,000 patients at Ministry of Health facilities, >50% of which enrolled in DSD since 2017.[30] Thus, there is widespread knowledge about the concept of DSD among HIV care providers at AMPATH-affiliated facilities. In the current standard-of-care PMTCT model, HIV services are delivered within integrated, undifferentiated maternal-child health (MCH) clinics rather than in HIV clinics.[2,27] Pregnant WLHIV are given monthly antenatal clinic (ANC) appointments which involve separate encounters with a clinician (i.e., clinical officer or physician), nurse, and mentor mother (i.e., peer counselor). Postpartum WLHIV and their infants maintain monthly, co-scheduled postnatal clinic (PNC) appointments through 12 months post-delivery, followed by appointment spacing every 2–3 months through 18 months post-delivery and cessation of breastfeeding, when the child’s final HIV test is usually done. Mentor mothers provide adherence counseling, psychosocial support, and outreach. Viral load testing is recommended every 6 months for pregnant or breastfeeding women.[28] Routine visit frequency for pregnant women without HIV is based on the WHO-focused antenatal care (FANC) model, which recommends at least four visits at 8–12, 24–26, 32, and 36–38 weeks of pregnancy.[31] As such, the monthly ANC visit schedule for WLHIV superseded the four-visit FANC model for women without HIV. Routine child vaccinations are given during postpartum follow-up, scheduled at birth, 6, 10, and 14 weeks, and 6, 9, and 18 months, and maternal and child appointments are synchronized whenever possible.[27] Thus, there are approximately 20–23 monthly clinic appointments for WLHIV from enrollment in ANC at the beginning of the 2nd trimester through 18 months post-delivery and 14–15 appointments for HIV-exposed infants from delivery through 18 months.
Population
Healthcare providers were eligible if they were clinicians, nurses, or mentor mothers =18 years of age who provided PMTCT services at either of two AMPATH-affiliated facilities, Moi Teaching and Referral Hospital (MTRH) and Huruma Sub-District Hospital (HSDH). MTRH is a large, tertiary referral hospital in an urban area of Eldoret serving an urban population. HSDH is a small, primary facility in a peri-urban area bordering Eldoret. Viral suppression is approximately 88–92% during pregnancy and the early postpartum period among women on ART for at least 3 months in these clinics.[18,32]
Twelve eligible providers were recruited in person by a research assistant, stratified by provider type, clinic, and facility. Providers completed study procedures outside of work hours.
Data Collection and Management
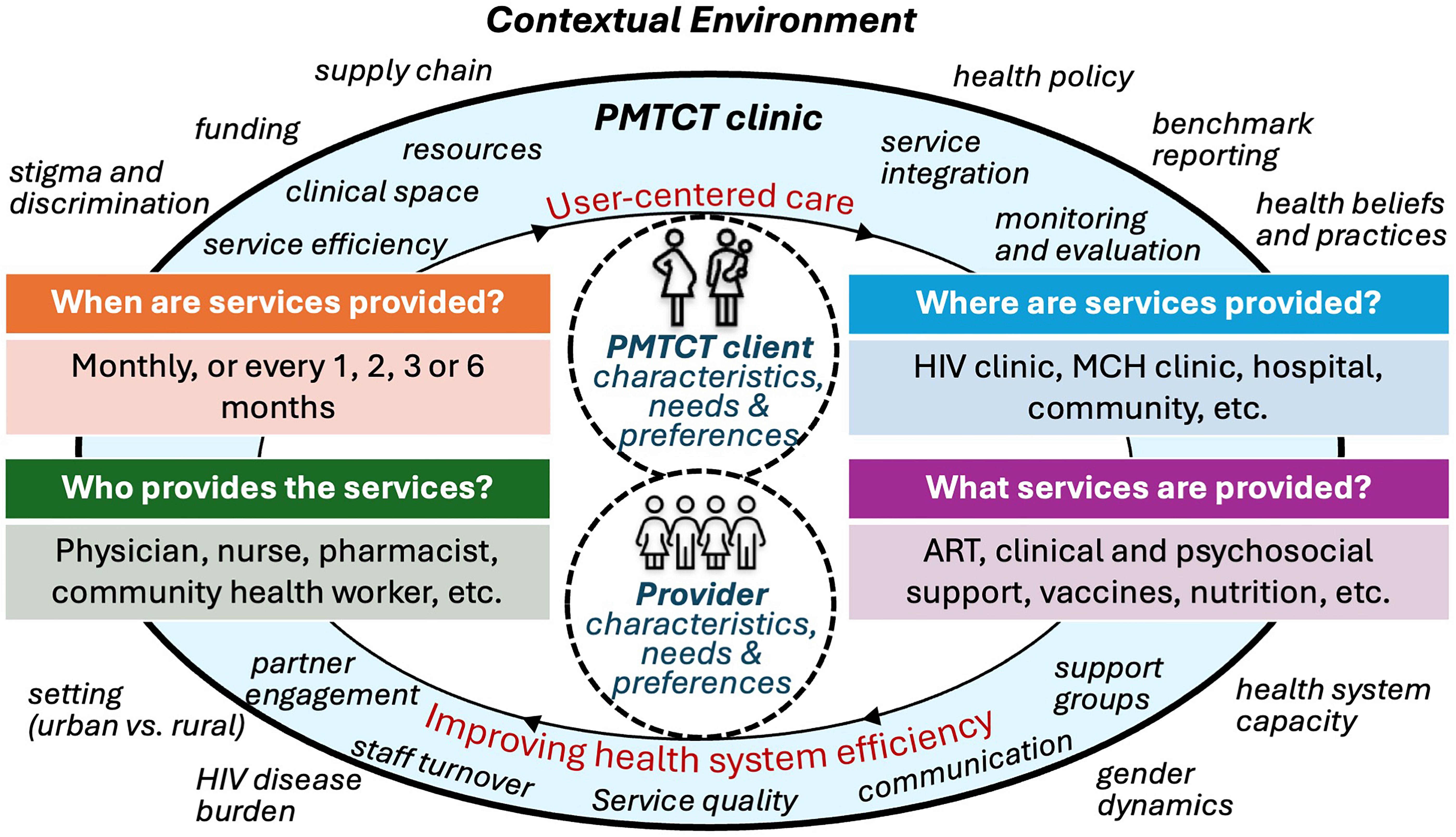
Data were collected through in-person, in-depth interviews with a Kenyan research assistant, administered using paper-based interview guides and audio-recorded. The interviewer took field notes relating to context and elements that could not be captured by audio. The interview guides were designed in English and translated into Kiswahili with back translation to ensure translation integrity. The guides were structured by the following domains: (1) perspectives on DSD for PMTCT; (2) characteristics of stable/unstable clients; and (3) strategies to improve service quality and efficiency, which relate to the aforementioned two core principles of DSD—providing client-centered care and improving health system efficiency [Figure 1]. Questions were contextualized according to clinic type (ANC versus PNC) and covered the four WHO's building blocks of DSD: when, where, and who provides services and what services are provided.[29]
Interviews were conducted in private rooms at the facilities and lasted approximately 60 minutes each. Audio recordings were transcribed and translated verbatim from Kiswahili to English by a trained translator. Identifying details were removed prior to analysis. Quality checks were performed by one of the Kenyan investigators (L.M.) by comparing random sections of audio with corresponding transcriptions and translations.
Statistical Analysis
Three investigators (J.H., J.C., and E.W.) established a coding framework using codes defined a priori based on PMTCT and DSD literature and our prior qualitative work exploring retention in care for PMTCT clients.[19] Codes were refined, and new codes were developed inductively using axial and selective coding during analysis. Coding was performed independently by two investigators (J.H. and J.C.) using analytic memos to ensure consistency and resolve differences through discussion with another investigator (E.W.). Inductive codes were discussed among two investigators (J.H. and J.C.) before adding them to the codebook. We used NVivo software (Melbourne, Australia) for analysis. Illustrative quotes were selected to highlight themes. Our reporting follows SRQR guidelines.[33]
RESULTS
Study Participants
Twelve providers were enrolled from September to October 2021. No individuals approached refused to participate. Overall, 10 (83%) were female, with a median age of 40 years (interquartile range [IQR]: 37–44 years), with a median of 7 years (IQR: 6–8 years) of experience delivering PMTCT services. Participants were equally distributed by facility (6 from MTRH and 6 from HSDH), clinic (6 from ANC and 6 from PNC), and provider type (4 clinicians, 4 nurses, and 4 mentor mothers). Most interviews were in English; mentor mothers used mixed English/Kiswahili.
Perspectives on Implementation of DSD for PMTCT
Providers were familiar with the concept of DSD, and most (9 of 12) felt that DSD should be available to PMTCT clients. Less frequent clinic visits were a primary benefit of DSD by easing healthcare burdens. One participant reflected on the challenge of monthly visits during pregnancy:
“I know antenatal you cannot fix monthly [visits], it is costly and the transport is a challenge. Assuming they are working, asking for permission from work is hectic every month.” (antenatal nurse, MTRH)
When asked how DSD would be received by PMTCT providers, one said:
“They will take it very positively. For example, for me, it will reduce my monthly workload.” (clinician, MTRH)
When asked to elaborate on how DSD could be implemented for stable PMTCT clients, one provider suggested decreasing ART refill frequency, saying:
“You see for those who are stable, you can dispense medication, say for three months. So even if they come for the ANC clinic in between, they don’t necessarily need to queue for dispensation. She just receives the ANC services and then she goes home.” (clinician, HSDH)
Referencing postnatal mothers, this same clinician said:
“She just takes a refill, maybe a longer duration. This other in-between when they bring the baby for immunization, she will not need to be attended to herself.” (clinician, HSDH)
All providers felt the risk of vertical transmission during pregnancy and breastfeeding required ’close monitoring’ and limited the adaptation of DSD for stable PMTCT clients. As one said:
“In PMTCT we are talking about a baby who is in the stomach and needs constant follow-up. Postnatal we are talking about a baby who has been born and needs constant follow-up. It is not just about the mother. I don’t think it will work or be beneficial in PMTCT; it will bring more harm than good.” (mentor mother, MTRH)
This woman explained the psychological impact vertical transmission had on providers:
“It is unfair to let this lady go to for like two or three months then you come and have a positive baby. It also affects us psychologically when you break news to a mother that the baby is positive.” (mentor mother, MTRH)
Providers also worried DSD could undermine retention given less frequent clinic appointments, saying:
“We also find the ones we are spacing for those two to three months they are also the most troublesome because they don’t keep their appointments.” (mentor mother, MTRH)
This risk was emphasized during late postpartum. As this provider said:
“And then after the one and a half test [i.e., infant HIV test at 18 months] we discharge the baby from the clinic yet we are supposed to see the mother up to two years. It becomes difficult to convince the mother to regularly come to the clinic when we discharge the baby when her main concern is the baby. Retaining them becomes an uphill task.” (mentor mother, MTRH)
The activities defining ’close monitoring’ included monitoring of the mother’s pregnancy progression, ART adherence, viral load, and the infant’s HIV prophylaxis, HIV testing, immunizations, and growth/development. As one provider said:
“For example, in ANC these mothers need close monitoring because of complications related to pregnancy. Secondly, there is an HIV-exposed infant who we are expecting who also needs close monitoring, so long TCA will not assist that mother [’TCA’ is an acronym for “To Come Again", which represents the duration of time until the client’s next scheduled date to come again to the clinic]. If you request for a longer TCA, in case of any problem, you will not notice.” (clinician, HSDH)
The concept of ’close monitoring’ extended to provider-patient relationships. Providers were asked whether a client would need to see a mentor mother during each clinical encounter as per the standard of care. While most did not feel this was necessary, one mentor mother cited the relational benefits of doing so:
“We always wish to be in contact with all the clients that we see irrespective of whether they are stable or they are not stable, it is good to be in touch with them. You can’t say that now that this lady is stable, I don’t need to [see her].” (mentor mother, HSDH)
Another mentor mother noted that close contact with expectant mothers facilitated relationships that could enhance care quality. A mentor mother explained,
“We have a calendar called expected date of delivery – EDC register – where we have an expected date. So, as we are filling the EDC, we call [the mother] ’Did you give birth?’ That small talk, ’Ooh, congratulations!’, so they will never forget to come for the first PCR in 6 weeks. ’How many kilos is the baby? You have a boy?’ They love this talk. It makes them feel very comfortable and happy.” (mentor mother, MTRH)
Another provider noted the need to maintain contact with clients in case they miss a scheduled appointment:
“Even if she is stable when they come on the scheduled visits, they should meet a mentor mother. Remember they are updating the locators, we also want the mentor mothers to bond with them a little bit.” (mentor mother, MTRH)
Postpartum visit spacing was limited by factors including the infant HIV testing and immunization schedules, growth monitoring, and antiretroviral supply. Several providers stated that DSD should not be introduced until the infant tests HIV negative after breastfeeding cessation. Reflecting this, one clinician said:
“If it will work, it will, after maybe 18 months confirming the antibody test negative, and maybe if she stopped breastfeeding we can give her even up to three months [until the next return visit]. Because we are sure now this child is HIV negative; we are sure the risks have reduced considering not breastfeeding.” (clinician, HSDH)
When asked about altering the monthly visit schedule for stable clients, one clinician cited the need to consider the infant immunization schedule:
“We would say any adjustment will occur around one year because there is that immunization at 9 months, the measles. But for the first 6 months there is no way you can change their visits.” (clinician, MTRH)
Providers also noted challenges adapting DSD to PMTCT given the need to follow county health reporting requirements, antiretroviral supply, and dose adjustments of infant prophylaxis. One provider said:
“It is tricky because like for the medication, the prophylaxis for the baby, they need to review the dosing based on weight.” (clinician, MTRH)
Perspectives about Characteristics of Stable and Unstable Clients
Suggested criteria to define stable clients included age over 18 or 20 years, higher socioeconomic status, and residing near the health facility. One provider noted that evaluating a client’s home could help identify their clinical status, saying:
“By doing home visits for them; everyone should be identified by how they are living.” (mentor mother, HSDH)
Suggested clinical criteria defining unstable clients included body mass index (i.e., BMI <18.5 indicating malnutrition), comorbidities (e.g., diabetes, hypertension, mental illness, and substance use disorders), obstetric history (e.g., multiple gestation pregnancies, prior c-section), and family history (e.g., having a prior HIV-positive child). HIV-related criteria included HIV viremia, non-adherence to ART and scheduled clinic appointments, receipt of 2nd- or 3rd-line ART, or opportunistic infections (e.g., tuberculosis and cryptococcal meningitis). One provider highlighted the interconnectedness of these criteria:
“Unstable is anybody who does not keep appointments, because keeping your appointments goes hand-in-hand with your [ART] adherence. When you miss appointments, chances are high that you might miss some doses. An unstable person for us is one who does not keep his or her appointments and viral load is not suppressed it is high.” (mentor mother, MTRH)
Timing of HIV diagnosis relating to pregnancy, prior (i.e., “known positive”) or during pregnancy (i.e., “newly positive”), was also a suggested eligibility criterion for DSD. One provider said:
“I would say a ’known positive’ should be a stable client, unlike a ’newly positive’ who is not stable who still asking themselves a lot of questions about their [HIV] status.” (postnatal nurse, MTRH)
One provider linked newly positive status to non-disclosure of HIV diagnosis, which was linked to non-retention, saying:
“The retention challenge is the new clients who still require more counseling, especially those who come from far, the issue of disclosure. Many of those who we have identified to have challenges in retention are the newly diagnosed.” (mentor mother, MTRH)
Non-disclosure was linked to suboptimal adherence. As another provider said:
“We also consider the ones who have not disclosed because they are at a risk. We become concerned about where they are keeping their medication, and how they are taking their medication.” (mentor mother, HSDH)
Providers highlighted that a client’s pregnancy/breastfeeding status should be a consideration in characterizing stable/unstable clients due to the risk of vertical transmission, saying:
“Postpartum is risky because this mother is also breastfeeding the child, so with a high viral load it requires close monitoring.” (mentor mother, HSDH)
Another provider emphasized the importance of avoiding mixed feeding, which can increase the risk of vertical transmission during breastfeeding:
“For postnatal, it does not depend on the mother it depends on the age of the baby. For the first 6 months where we want to determine the exclusivity of being breastfed, we follow up monthly.” (mentor mother, MTRH)
The infant’s appearance was suggested as a way to define socioeconomic vulnerability:
“When a mother comes with her child and you look at the child, you will know that how this baby is feeding is not good. That is through nutrition. Another thing is how the baby has been dressed when coming to the clinic. You will know for sure that even wherever this mother is coming from she is not stable.” (mentor mother, MTRH)
Providers noted the terms “stable” and “unstable” were potentially stigmatizing. One clinician noted that it was not clients who were unstable, but rather the conditions which increased their vulnerability. One provider said:
“When you say ’unstable’ you generalize a victim. It depends on the unstable. What makes this mother to be unstable? Is it her medical condition, adherence issues you will base that duration or TCA, depending on the condition?” (clinician, HSDH)
Strategies to Improve PMTCT Service Quality and Efficiency
Providers recommended strategies to improve service quality and efficiency that could be incorporated into DSD. Their responses revolved around six themes: integration of HIV and other health services, clinical space, provider-client communication, male partner engagement, clinic waiting time, and facility-based support groups.
Providers unanimously recommended the integration of contraception and reproductive health services (e.g., cervical and breast cancer screening) into routine PMTCT care, noting these services were currently accessed at separate clinical areas. One said:
“We do not do the family planning here [i.e., in the PNC clinic] because if we were to do it here, [women] have to pay the hospital fee for the service… If you tell her that you are going up there [i.e., to the family planning clinic] to get the method, she becomes discouraged.” (mentor mother, MTRH)
The need for more physical space in ANC and PNC was noted as a barrier to service integration and was needed to protect privacy and confidentiality. One provider said:
“You can see we are in a hall, and in a hall, you cannot get exact things from the client. There are some things that she will hide. So, we are not giving the ideal counseling the way it is supposed to be done.” (clinician, HSDH)
One provider explained the challenge pregnant WLHIV face in maintaining privacy:
“They have divided [ANC] with a curtain. You find that if someone is with her neighbor or they are coming in the same area, she maybe attended with vaccination and then goes outside and avoid going in there even though they know they should be going inside there because she has come with a friend, neighbor and so forth. She will not go in there because they will go and ask her many questions.” (mentor mother, MTRH)
Providers suggested means of enhancing communication as ways to improve service quality. One noted the importance of establishing relationships with clients, saying:
“We equip ourselves to show these mothers love… We give them the services, we give them drugs and love them; by loving them, we give them a call; ’mummy, how are you doing? How did the drugs take you? How is life taking you?’… You don’t need to wait for them until they come on their clinic day.” (mentor mother, HSDH)
She elaborated on this theme by citing the value of home visits, saying:
“You know you will know one who is sick, sincerely sick, she does not even have fare for coming to the clinic, and she is completely unable. So if the program implements that one where people would be visited on home visits that will improve [service quality]… It will be very good because you will find a mother who is very sick and so much overwhelmed in the house and because now she will not tell her neighbor… but, for us, they know we are their people and when we go there we may know their problems and we will bring them to hospital and they will get helped.” (mentor mother, HSDH)
Insufficient staff and social services were communication barriers and included the problem of staff turnover and retraining needs. As one said:
“The problem here is that when you train somebody on PMTCT you find after a few months he or she is being rotated. It is beyond us, you find resources were used on this person and now he or she is being removed from where he or she would have rendered the service and taken elsewhere and then you get a totally green person.” (mentor mother, MTRH)
Male partner engagement and male preventive health services (e.g., prostate cancer screening) were suggested as ways to enhance service quality. Explaining the partner’s role in Kenya’s sociocultural context, one provider said:
“Most of the male partners are the breadwinners in the families and some claim it is their day to go and fend for their families. If we can incentivize them in terms of monetary and foodstuffs, they can be motivated.” (clinician, HSDH)
Within this theme, a nurse suggested that priority could be given to women who come to the clinic with their partners:
“When they come, they are always in a hurry. That is why when we see them, we tell them ’couples, go in’ and we attend to them.” (antenatal nurse, HSDH)
Providers at both facilities acknowledged long clinic wait times and suggested ’fast-track’ encounters for stable clients could improve efficiency. One nurse said:
“My thinking is that I would rather stay with one client for one hour and help that client forever unlike clearing and forwarding and I am losing almost all of them.” (postnatal nurse, MTRH)
A clinician cautioned that DSD for WLHIV could increase disclosure risk, saying:
“It will bring stigma. Even as they come to the clinic they ask, ’why have you gone there and this one is going here?’” (clinician, MTRH)
Scheduling stable and unstable clients on separate clinic days was suggested by several providers:
“In a week we allocate some days for the stable clients and some for the unstable clients… We put the unstable clients, the ones we see still have issues (the newly diagnosed) we want them to come on Friday, those with high viral loads to come on Friday, and the children whose mother has gone to school and the child turned [HIV-positive] and the caretaker is the one looking after the baby we see them on Friday.” (postnatal nurse, MTRH)
Facility-based support groups for PMTCT clients were also raised by providers to enhance service quality. As one said:
“If they were together, it would have been very good, because all mothers would have shared their views. Those who have taken care of their babies and those who are expecting babies… If they were together and this mother talks about how she has taken care of her baby, you see this pregnant one will understand that if I also give birth to my baby, I will take care of her in the same way and she will be [HIV] negative.”
DISCUSSION
In this study, we describe the perspectives of Kenyan healthcare providers on the implementation of DSD for PMTCT clients. Providers expressed support for the core principles of DSD but had several concerns that can be summarized using the elements needed to provide client-centered ART delivery: context, specific populations, and clinical characteristics [Figure 1].3
The context in which DSD is being implemented is an important consideration and includes urban/rural settings, epidemic type (generalized/concentrated), and HIV burden (high/low), among other factors.[3] Providers had similar views on the benefits of DSD but cited barriers to improving service quality and efficiency in their clinics. Limited physical space was a key theme for providers at MTRH and was linked to suboptimal integration of HIV and family planning services and risk of disclosure. These themes were less prevalent among HSDH providers. Decentralizing services through group- and community-based DSD could help decongest clinics with limited staff and space at MTRH.[12] However, this model may not be practical for facilities like HSDH. Our prior research also indicated that group-based DSD may not be desirable to PMTCT clients in a setting with low HIV prevalence due to the perceived risk of stigma and disclosure.[19,34] The adaptation of DSD to PMTCT settings will need to consider inter-facility contextual factors in determining the optimal model for a given clinic.
The population for whom DSD is implemented must also be considered. Providers held positive views about the benefits of DSD but expressed concern that reduced visit frequencies and ART refills would worsen vertical transmission, potentially limiting DSD’s application to the PMTCT population. Incorporating implementation strategies for DSD such as data monitoring and program evaluation, and audit and feedback with providers to review safety and effectiveness data when implementing a DSD for PMTCT could help address this concern.
We also highlight important considerations in determining PMTCT clients’ eligibility for DSD. Most criteria defining “stable” clients aligned with DSD guidelines for non-pregnant or breastfeeding PLHIV.[3,35] However, providers suggested additional criteria relating to pregnancy history and socioeconomic status. Combined with other measures that predict the risk of adverse pregnancy and postpartum outcomes (e.g., postpartum depression), these additional criteria suggest a DSD model for PMTCT may require unique criteria compared to current DSD guidelines for other populations.
Our findings are further contextualized within current guidelines for DSD implementation in other African countries. In South Africa, for example, where community “adherence clubs” are widely implemented, clinically stable postpartum women randomized to community adherence clubs had a 29% reduction in viremia through 24 months postpartum compared to women attending standard postpartum clinics.[36,37] Despite limited evidence, some countries have incorporated elements of DSD in their national HIV treatment guidelines. In Zimbabwe, pregnant and breastfeeding WLHIV may receive HIV clinic visits and ART refills every 6 months while adhering to routine ANC and postnatal care.[38] WLHIV enrolled in DSD who become pregnant may remain in differentiated HIV services or transfer to an integrated ANC/HIV clinic with ART refills every 6 months. In Ghana, stable WLHIV (i.e., on ART =6 months, viral load <50 copies/mL) who become pregnant may also receive community- or facility-based differentiated HIV services while attending routine ANC.[39] Other countries have incorporated multi-month ART dispensing for pregnant and breastfeeding women into national guidelines.[40,41,42] This guidance highlights the growing interest in DSD for PMTCT populations and the need for data to understand the comparative effectiveness of these models.
Our study was conducted during the COVID-19 pandemic when many HIV programs adapted by expanding DSD, which may have influenced providers’ views on DSD versus pre-pandemic.[9] Our small sample size also may not represent the perspectives of all PMTCT providers in the region, including in more rural areas, though thematic saturation was achieved among those sampled. Our study focuses on front-line healthcare providers and does not include the perspectives of patients or other stakeholders. Social desirability bias may have impacted providers’ willingness to provide unfavorable perspectives on PMTCT services.
CONCLUSION AND GLOBAL HEALTH IMPLICATIONS
Filling the gap in guidance on DSD for PMTCT will require adaptations to DSD that are responsive to providers’ concerns and the uniqueness of the pregnancy-postpartum continuum. Optimal guidance may vary across settings based on contextual, clinic, and client-level factors. More research is needed to understand the effectiveness of various DSD models for PMTCT and the perspectives of broader PMTCT stakeholders, including patients and policymakers, on its implementation.
Key Messages
1. Differentiated service delivery (DSD) has been widely adopted in sub-Saharan Africa, but DSD for women and infants enrolled in the prevention of vertical HIV transmission services is lacking. 2. Providers in western Kenya hold positive views about the potential benefits of DSD for pregnant and postpartum women living with HIV and their infants. 3. Filling the gap in guidance for DSD implementation for this population will require adaptations to the DSD model that are responsive to providers’ concerns and the unique aspects of the continuum of services for pregnant and postpartum women living with HIV and their infants.
Acknowledgments
The authors thank the clinical staff at the study facilities for helping to make this study possible. We thank the clinical staff at Moi Teaching and Referral Hospital and HSDH in Kenya for helping to make this study possible.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest: Unrelated to the current work, Dr. Gregory Zimet has served as an external advisory board member for Pfizer and Moderna, and as a consultant to Merck, has received investigator-initiated research funding from Merck administered through Indiana University and serves as an unpaid member of the Board of Directors for the Unity Consortium, a nonprofit organization that supports adolescent health through vaccination. The other authors declare no competing interests. Financial Disclosure: Nothing to declare. Funding/Support: This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (Grant Numbers K23HD105495 to J.H. and K23HD109056 to J.C.). Ethics Approval: The study was approved by the Moi University/Moi Teaching and Referral Hospital Institutional Research and Ethics Committee (#IREC/2021/51) in Kenya and the Indiana University Institutional Review Board (#10925) in the United States. A research permit was also granted by the National Commission for Science, Technology, and Innovation (NACOSTI, #702668) in Kenya. Declaration of Patient Consent: The authors certify that they have obtained all appropriate patient consent. Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation: The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Achieving viral suppression in 90% of people living with human immunodeficiency virus on antiretroviral therapy in low- and middle-income countries: Progress, challenges, and opportunities. Clin Infect Dis. 2018;66((10)):1487-91.
- [Google Scholar]
- Key considerations for differentiated antiretroviral therapy delivery for specific populations: Children, adolescents, pregnant and breastfeeding women and key populations. Geneva: WHO;
- Differentiated care for HIV: A decision framework for antiretroviral therapy delivery.. Geneva: IAS;
- Differentiated service delivery. Updated. [cited 2022 May 2]. Available from: http://www.differentiatedservicedelivery.org
- [Google Scholar]
- Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528((7580)):S68-76.
- [Google Scholar]
- Adherence clubs and decentralized medication delivery to support patient retention and sustained viral suppression in care: Results from a cluster-randomized evaluation of differentiated ART delivery models in South Africa. PLoS Med. 2019;16((7)):e1002874.
- [Google Scholar]
- High rates of retention and viral suppression in the scale-up of antiretroviral therapy adherence clubs in Cape Town, South Africa. J Int AIDS Soc. 2017;20((Suppl 4)):21649.
- [Google Scholar]
- Cost of differentiated HIV antiretroviral therapy delivery strategies in sub-Saharan Africa: A systematic review. J Acquir Immune Defic Syndr. 2019;82(Suppl 3):S339-47.
- [Google Scholar]
- Silver linings: How COVID-19 expedited differentiated service delivery for HIV. J Int AIDS Soc. 2021;24((Suppl 6)):e25807.
- [Google Scholar]
- Differentiated HIV care in sub-Saharan Africa: A scoping review to inform antiretroviral therapy provision for stable HIV-infected individuals in Kenya. AIDS Care. 2018;30((12)):1477-87.
- [Google Scholar]
- Evidence for scale up: The differentiated care research agenda. J Int AIDS Soc. 2017;20((Suppl 4)):22024.
- [Google Scholar]
- Differentiated models of care for postpartum women on antiretroviral therapy in Cape Town, South Africa: A cohort study. J Int AIDS Soc. 2017;20((Suppl 4)):21636.
- [Google Scholar]
- UNAIDS data;. Updated December 4, 2019 [cited 2022 May 2]. Available from: https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data
- [Google Scholar]
- Investigating the implementation of differentiated HIV services and implications for pregnant and postpartum women: A mixed methods multi-country study. Glob Public Health. 2021;16((2)):274-87.
- [Google Scholar]
- A review of differentiated service delivery for HIV treatment: effectiveness, mechanisms, targeting, and scale. Curr HIV/AIDS Rep. 2019;16((4)):324-34.
- [Google Scholar]
- Retention in care and viral suppression in differentiated service delivery models for HIV treatment delivery in sub-Saharan Africa: A rapid systematic review. J Int AIDS Soc. 2020;23((11)):e25640.
- [Google Scholar]
- Achieving UNAIDS 90-90-90 targets for pregnant and postpartum women in sub-Saharan Africa: Progress, gaps and research needs. J Virus Erad. 2018;4((Suppl 2)):33-9.
- [Google Scholar]
- Retention in care and viral suppression in the PMTCT continuum at a large referral facility in western Kenya. AIDS Behav. 2022;26((11)):3494-505.
- [Google Scholar]
- A qualitative study of the barriers and enhancers to retention in care for pregnant and postpartum women living with HIV. PLOS Glob Public Health. 2021;1((10)):e0000004.
- [Google Scholar]
- No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015;61((11)):1715-25.
- [Google Scholar]
- No HIV transmission from virally suppressed mothers during breastfeeding in rural Tanzania. J Acquir Immune Defic Syndr. 2018;79(Sep 1)J Acquir Immune Defic Syndr. 2018;79((1)):e17-20.
- [Google Scholar]
- Donor government funding for HIV in low- and middle-income countries in 2020. Updated July 15, 2021 [cited 2022 Apr 14]. Available from: https://www.kff.org/global-health-policy/report/donor-government-funding-for-hiv-in-low-and-middle-income-countries-in-2020/
- [Google Scholar]
- UNAIDS data. [cited 2024 May 15]. Available from: https://www.unaids.org/en/resources/documents/2023/2023_unaids_data
- [Google Scholar]
- Fast-track-ending the AIDS epidemic by 2030. Updated November 18 [cited 2022 Jun 10]. Available from: https://www.unaids.org/en/resources/documents/2014/JC2686_WAD2014report
- [Google Scholar]
- Economic evaluation of differentiated service delivery models for HIV treatment in Lesotho: Costs to providers and patients. J Int AIDS Soc. 2021;24((4)):e25692.
- [Google Scholar]
- Guidelines on use of antiretroviral drugs for treating and preventing HIV in Kenya. Nairobi: NASCOP;
- [Google Scholar]
- Kenya HIV prevention and treatment guidelines. [cited 2023 Aug 16]. Available from: https://www.differentiatedservicedelivery.org/wp-content/uploads/Kenya-ARV-Guidelines-2022-Final-1.pdf
- [Google Scholar]
- Updated recommendations on service delivery for the treatment and care of people living with HIV. Geneva: World Health Organization; [cited 2023 Aug 15]. Available from: https://www.who.int/publications-detail-redirect/9789240023581
- [Google Scholar]
- AMPATH; [cited 2022 May 6]. Available from: http://www.ampathkenya.org
- National guidelines for quality obstetrics and perinatal care. [cited 2023 Aug 25]. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjn07yFvaWCAxWiJkQIHXnhB_4QFnoECAkQAQ&url=http%3A%2F%2F2Fguidelines.healthgo.ke%2F%23%2Fcategory%2F27%2F91%2Fmeta&usg=AOvVaw0FQ45Q0saxA7MiOL80i8Uw&opi=89978449
- [Google Scholar]
- Outcomes after loss to follow-up for pregnant and postpartum women living with HIV and their children in Kenya: A prospective cohort study. J Acquire Immun Defic Syndr. 2024;97((3)):242-52.
- [CrossRef] [Google Scholar]
- Standards for reporting qualitative research: A synthesis of recommendations. Acad Med. 2014;89((9)):1245-51.
- [Google Scholar]
- High HIV prevalence predicts less HIV stigma: A cross-national investigation. AIDS Care. 2018;30((6)):714-21.
- [Google Scholar]
- Improved virologic outcomes in postpartum women living with HIV referred to differentiated models of care. AIDS. 2022;36((15)):2203-11.
- [Google Scholar]
- ART adherence clubs: A long-term retention strategy for clinically stable patients receiving antiretroviral therapy. South Afr J HIV Med ((14)):48-50.
- [Google Scholar]
- Operational and service delivery manual for the prevention, care and treatment of HIV in Zimbabwe. AIDS & TB Programme; [cited 2023 Sep 2]. Available from: https://www.differentiatedservicedelivery.org/wp-content/uploads/MSF-Zim-OSDM-Nov2022-WEB2.pdf
- [Google Scholar]
- Differentiated service delivery for HIV in Ghana: An operational manual;. [cited 2023 Sep 2]. Available from: https://www.differentiatedservicedelivery.org/wp-content/uploads/DSD-GHANA_2022.pdf
- [Google Scholar]
- Interim guidance for provision of HIV services in the context of COVID-19 pandemic in Ethiopia. [cited 2023 Sep 2]. Available from: https://www.differentiatedservicedelivery.org/wp-content/uploads/ethiopia_COVID.pdf
- [Google Scholar]
- Memorandum: Guidance on provision of chronic care during the COVID-19 pandemic. [cited 2023 Aug 30]. Available from: https://www.differentiatedservicedelivery.org/wp-content/uploads/Memorandum-Chronic-care-during-Covid-19-22-April-1.pdf
- [Google Scholar]
- Guidelines for HIV prevention, treatment and care in Rwanda. [cited 2023 Aug 30]. Available from:https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKE2ahUKEwjbqrztvaWCAxVMH0QIHfCcAMkQFnoECBQQAQ&url=https%3A%2F%2F www.fdifferentiatedservicedelivery.org%2Fnational-policies%2F&usg=AOvVaw3WRI2zXkGzeT-BBc2d2dAg&opi=89978449
- [Google Scholar]






