Translate this page into:
Adherence to Combination Antiretroviral Therapy among Pregnant Women Enrolled in a HIV Prevention Program in Rural North-central Nigeria
∗Corresponding author email: muktar.aliyu@vumc.org
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective:
Adherence to combination antiretroviral therapy (ART) among pregnant women is essential to attaining the goal of eliminating mother-to-child HIV transmission. The objective of this study was to determine which factors affect adherence to ART among HIV-positive women enrolled in a large prevention of mother-to-child HIV transmission (PMTCT) trial in rural north-central Nigeria.
Methods:
The parent study included 372 HIV-positive pregnant women enrolled in a cluster-randomized control trial conducted at 12 health facilities in Nigeria between 2013 and 2015. This secondary analysis included HIV-positive women (and their infants) from the original trial with documented adherence data (n=210, 56.5%). The primary outcome was maternal adherence to ART, determined by self-report and based on the visual analogue scale (VAS) of a validated medication adherence tool. Participants with a VAS score of ≥ 95% were classified as adherent. We employed multivariate logistic regression to evaluate the predictors of maternal ART adherence in the study sample.
Results:
Approximately 61.0% of study participants (128/210) were adherent to ART. The majority of adherent participants (62.5%, 80/128) were enrolled in the trial intervention arm. The most common cited response for non-adherence was fear of status disclosure. Adherence to ART was associated with study arm (intervention arm vs. control arm, adjusted Odds Ratio (aOR) [95% CI]: 16.95 [5.30-54.23]), maternal ethnicity (Gwari vs. Other, aOR = 0.13 [0.05-0.38]), and partner HIV status (HIV-positive vs. unknown, aOR = 3.14 [1.22-8.07]).
Conclusion and Global Health Implications:
Adherence to ART among a cohort of pregnant women enrolled in a PMTCT trial in rural North-Central Nigeria was associated with trial arm, maternal self-reported ethnicity, and partner’s HIV status. Increased understanding of the interplay between these factors will enable the development of more targeted and effective interventions.
Keywords
HIV/AIDS
PMTCT
Combination antiretroviral therapy
Adherence
PMCTC
Nigeria
1. Introduction
1. Background of the Study
Advances in combination antiretroviral treatment (ART) have resulted in significant worldwide declines in HIV-associated mortality and morbidity.1,2 The majority of HIV-positive adults on treatment can expect to have near-normal life expectancy, especially if initiated on ART early in the course of their disease.3,4 These improvements in life expectancy are however, predicated on high levels of ART adherence, often at the ≥95% adherence threshold for successful viral suppression.5-7 Poor adherence to ART is associated with an increased risk of HIV transmission, treatment failure, suboptimal viral suppression and antiretroviral drug resistance.5,8-10
The adverse effects associated with poor ART adherence are equally applicable to pregnant women and their offspring. Suboptimal levels of ART adherence are associated with virological failure, poor maternal health outcomes and elevated rates of vertical HIV transmission.11,12 Adherence to effective ART is an essential component of programs for the prevention of mother-to-child HIV transmission (PMTCT), and can decrease vertical HIV transmission rates to <2% among non-breastfeeding women and <5% among breastfeeding women, while at the same time significantly reducing maternal and infant morbidity and mortality.13,14
As Africa’s most populated country (estimated population ~190 million), Nigeria has the fourth largest population of persons living with HIV worldwide.15 Nigeria also bears a significant proportion of the global burden of mother-to-child HIV transmission, in addition to the largest gap in uptake of PMTCT services.15,16 More than one-quarter of all perinatal HIV infections worldwide occur in Nigeria – an estimated 37,000 new mother-to-child HIV transmissions were reported in 2016 alone.15
1.2. Objectives of the Study
In 2012, Vanderbilt University received US National Institutes of Health funding to conduct an implementation science trial in rural north-central Nigeria. The purpose of the cluster-randomized trial was to examine the impact of an innovative package of PMTCT services (including task shifting, male partner engagement, point-of-care CD4+ testing and postpartum integration of mother-infant care) on selected outcomes, primarily ART uptake and mother-infant retention in care at 6- and 12- weeks postpartum.17,18 Although the parent PMTCT clinical trial reported an increase in uptake of ART among the study population, a formal evaluation of ART adherence in the study population has not been performed. Without optimal ART adherence, higher ART coverage rates will not be effective at reducing mother-to-child HIV transmission.11,12 Considering the paucity of published work on ART adherence among pregnant women in Nigeria,19 the importance of ART adherence to optimal PMTCT outcomes,12-14 and the high burden of vertical HIV transmission in Nigeria,16 we conducted a secondary data analysis to determine which factors predict ART adherence among pregnant women enrolled in our parent trial. Evidence from this study will inform the development of effective interventions that could be implemented in similar settings to increase ART adherence and decrease mother-to-child HIV transmission rates.
1.3. Specific Aims and Hypothesis
The specific aim of this study was to identify the factors associated with adherence to ART among mothers enrolled in a PMTCT implementation research clinical trial in rural Nigeria. We hypothesize that maternal education, HIV status disclosure to the partner, partner involvement in PMTCT, and maternal retention in care would be associated with treatment adherence.
2. Methods
2.1. Study Variables
This study is a secondary analysis of data from a 12-site cluster-randomized control trial conducted between 2013 and 2015 in rural North-Central Nigeria.17,18 Study participants were HIV-positive women who presented for routine antenatal care and/or delivery (and their infants) and who met the following criteria: (i) unknown HIV status at presentation; (ii) history of antiretroviral prophylaxis or treatment, but not currently receiving any antiretroviral medications at the time of presentation; and/or iii) known positive HIV status, but had never received antiretroviral treatment. The study sites were matched by antenatal clinic volume, HIV patient volume, degree of urbanization of the area and site accessibility, then randomly assigned to the control (standard-of-care) or treatment (integrated PMTCT service package) arm. The integrated PMTCT service package included transitioning of PMTCT tasks to trained midwives (task shifting), same-day point-of-care CD4+ testing, integrated mother-infant dyad HIV care, increased male partner engagement, and peer mentors for male partners. Participants in both intervention and standard of care sites received group health education, opt-out HIV testing, same-day HIV test results, infant feeding counselling, home-based care services, infant prophylaxis, early infant diagnosis (DNA PCR testing), and linkage to family spacing services, if desired.
2.1.1. Cohort description
For this secondary analysis, we included all HIV-positive women (and their infants) from the original PMTCT trial who had documented adherence measures, specifically Medication Adherence Self-Report Inventory (MASRI) questionnaire data, including visual analog scale (VAS) scores (Figure 1). The VAS is a measurement tool that consists of a rating scale depicted on a horizontal line. The respondents were asked to provide a numerical self-rating of their ART adherence by marking a point along the scale that corresponded with their estimated adherence over the past month prior to their visit. We excluded HIV-positive women (and their infants) who either had undocumented MASRI data or had incomplete MASRI data with missing VAS scores.
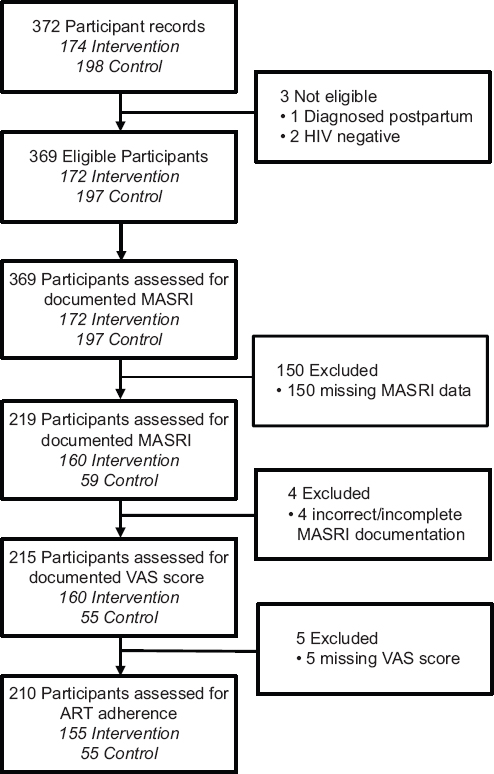
- Flowchart depicting study activities, Niger state, northcentral Nigeria
2.1.2. Study variables
The data collection process for the parent trial has been described earlier.17,18 Briefly, all demographic, PMTCT, and HIV care and treatment data were routinely collected and documented in the WHO HIV Care Card and/or patient management and monitoring forms. Select clinical and laboratory data were extracted from these forms and entered by research assistants into CAREWare™ (JPROG, New Orleans, LA, USA), an electronic medical record system adapted at supported sites, and REDCap™ (Research Electronic Data Capture), a secure, web-based software platform designed to support data capture for research studies.20 All participant data were de-identified, entered in REDCap™ and assigned a unique identifier to enable analysis of individual outcomes.
For this study, we extracted data from REDCap™ pertaining to demographics, immune status (i.e., CD4+ cell count values), postpartum visits, partner information, infant outcomes and responses from a self-reported adherence questionnaire (the Medication Adherence Self Report Inventory, MASRI).21 Partner information that was collected included: partner involvement (coded “yes” if participant was accompanied by male partner to at least one antenatal care visit); partner HIV status disclosure (coded “yes” if the participant’s partner had disclosed his HIV status to the participant); and partner HIV status (positive, negative or unknown). Partner HIV status was obtained via participant self-report or from male partner HIV testing performed at the antenatal clinics. Infant outcomes data included information on infant survival (alive or dead) and infant HIV status. Infant death included stillborn, miscarriage, and neonatal death. Date of death was based on clinic records and maternal/family member report. All data were coded as categorical variables, except for age, parity, years of education and CD4+ cell count.
The MASRI questionnaire administered in the parent trial was a modified version of the 12-item MASRI, published in 2002.21 The first section of the MASRI included a series of questions about missed ART doses. Participants were asked to quantify the number of doses missed the day before, 2 days ago, 3 days ago, and over the last 2 weeks. The modified MASRI questionnaire consisted of two different VAS measurements. The VAS questions were followed by “a permissive statement in order to encourage accurate responses” and reduce the potential for response bias.21 For the purposes of this study, we only utilized the VAS score from the question which asked about the percentage of medication taken within the past month (0-100%). The omitted VAS question asked about doses taken at the correct time, which does not provide as much of a meaningful understanding of adherence as doses taken within the past month. Participants were also asked to indicate the last time a dose was missed and the reasons for missed doses. The second part of the questionnaire addressed disclosure and partner HIV status. Participants were asked if they had recently disclosed their status to anyone, if their partner was aware of his HIV status, and if their partner had disclosed his status to the participant. If the participant was aware of their partner’s status, they were asked to indicate what their partner’s HIV status was.
The primary outcome for this study was maternal ART adherence (classified as adherent or non-adherent). For the purposes of this study, adherence was determined by self-report, based on the MASRI questionnaire. We used the VAS score as the sole measure of adherence in this study because of its ease of interpretation and literature that support its reliability and validity.21-23 Participants were classified as adherent if their VAS score was ≥95%, and as non-adherent if their VAS score was <95%. The choice of this 95% threshold is consistent with the definition of optimal ART adherence in the literature and based on the greater likelihood of favorable treatment outcomes at this level.24-26 In the parent trial, some participants completed the MASRI questionnaire more than once and in some instances responses to questions were left blank. We calculated the median VAS scores for participants with more than one VAS score recorded, and assigned the median VAS score as the single adherence score for this analysis.
2.2. Statistical Analysis
Data were extracted from REDCap™ and imported into IBM® SPSS® Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA) for analysis. We employed univariate analyses and descriptive statistics to summarize the frequencies and percentages of independent variables. MASRI questionnaire responses, not including the VAS score, were analyzed descriptively using measures of frequency. Variable categories with very low frequencies (0 or 1) were either collapsed into other subgroups or not included in the analysis. Continuous variables were categorized into subgroups for ease of interpretation. Marital status was not included in the analysis because almost 100% of participants were married, which resulted in very low frequencies in the remaining categories.
We employed bivariate analysis using Pearson’s chi-square test to identify statistically significant relationships between categorical independent variables and the outcome variable (ART adherence). The independent variables that were found to be significantly associated with ART adherence at a significance level of 0.10 on bivariate analysis were then inputted into a multivariate regression model. As the unit of analysis (women) was unmatched, we performed unconditional multivariate logistic regression to evaluate the associations between the independent variables and adherence. The significance level for logistic regression was set at 0.05.
2.3. Ethical Approval
The parent PMTCT trial received approval from the Nigeria National Human Research Ethics Committee and the Vanderbilt University Institutional Review Board (IRB #121257). The secondary analysis of adherence data received approval from the Institutional Review Board of Meharry Medical College (IRB #18-11-870).
3. Results
3.1. Sociodemographic Characteristics
A total of 210 of 372 (57%) HIV-positive women and their infants that were recruited in the parent clinical trial met the inclusion criteria for this secondary analysis. Sixty-one percent of participants (128/210) were adherent to ART. Participants who met the inclusion criteria attended 9 of the 12 trial clinics, and the majority were enrolled in the study intervention arm (73.8%, n=155) (Table 1). The majority of participants were married (98.1%, not shown) and homemakers (62.4%). The median age (interquartile range, IQR) of participants was 28 years (24-30). One in three participants (33.8%) had no formal education. Most women reported belonging to an ethnic group other than Hausa-Fulani, Gwari, and Nupe. The most common ethnicities in the “Other” group were Igbo, Yoruba, and Kambari. The median (IQR) CD4+ cell count of participants was 408 cells/mm3 (267-651). Most participants had a CD4+ cell count greater than 200 cells/mm3 (72.9%), with 45% of them being in the 200-499 cells/mm3 range (Table 1).
| Variables | Combined, N=210 No. (%) | Adherent, n=128 No. (%) | Not adherent, n=82 No. (%) | P-value |
|---|---|---|---|---|
| Study arm | <0.001 | |||
| Control | 55 (26.2) | 48 (37.5) | 7 (8.5) | |
| Intervention | 155 (73.8) | 80 (62.5) | 75 (91.4) | |
| Maternal age (years), median (IQR) | 28 (25-31) | 28 (25-31) | 26 (22.5-29.5) | 0.184 |
| Maternal age (years) | 0.307 | |||
| 17-24 | 56 (26.7) | 31 (24.2) | 25 (30.5) | |
| 25-29 | 72 (34.3) | 41 (32.0) | 31 (37.8) | |
| 30-35 | 67 (31.9) | 47 (36.7) | 20 (24.4) | |
| 36-49 | 15 (7.1) | 9 (7.0) | 6 (7.3) | |
| Years of education, median (IQR) | 6 (0-12) | 6 (0-12) | 4 (0-12) | 0.660 |
| Schooling, n (%) | 0.134 | |||
| Started primary | 20 (9.5) | 11 (8.6) | 9 (11.0) | |
| Completed primary | 32 (15.2) | 25 (19.5) | 7 (8.5) | |
| Secondary | 46 (21.9) | 30 (23.4) | 16 (19.5) | |
| Post-secondary | 8 (3.8) | 6 (4.7) | 2 (2.4) | |
| Qur’anic | 32 (15.2) | 19 (14.8) | 13 (15.9) | |
| None | 71 (33.8) | 36 (28.1) | 35 (42.7) | |
| Missing | 1 (0.5) | 1 (0.8) | -- | |
| Employment status | 0.183 | |||
| Employed | 7 (3.3) | 5 (3.9) | 2 (2.4) | |
| Unemployed | 55 (26.2) | 35 (27.3) | 20 (24.4) | |
| Homemaker | 131 (62.4) | 74 (57.8) | 57 (69.5) | |
| Other | 17 (8.1) | 14 (10.9) | 3 (3.7) | |
| Ethnicity | 0.006 | |||
| Hausa-Fulani | 38 (18.1) | 21 (16.4) | 17 (20.7) | |
| Gwari | 55 (26.2) | 29 (22.6) | 26 (31.7) | |
| Nupe | 45 (21.4) | 24 (18.7) | 21 (25.6) | |
| Other | 60 (28.6) | 48 (37.5) | 12 (14.6) | |
| Missing | 12 | 6 (4.7) | 6 (7.3) | |
| Parity, median (IQR) | 2 (1.0-3.3) | 2 (1.0-2.0) | 2 (0.5-3.5) | |
| Parity | 0.228 | |||
| 0-3 | 158 (75.2) | 99 (62.7) | 59 (37.3) | |
| 4-6 | 42 (20.0) | 26 (61.9) | 16 (38.1) | |
| 7+ | 10 (4.8) | 7 (70.0) | 3 (30.0) | |
| CD4+ cell count/mm3, median (IQR) | 408 (267-651) | 380 (204-555) | 424 (249-600) | 0.495 |
| CD4+ cell count/mm3 | 0.598 | |||
| <50 | 3 (1.4) | 1 (0.8) | 2 (2.4) | |
| 50-100 | 17 (8.1) | 10 (7.8) | 7 (8.5) | |
| 201-499 | 94 (44.8) | 60 (46.9) | 34 (41.5) | |
| ≥500 | 59 (28.1) | 33 (25.8) | 26 (31.7) | |
| Missing | 37 | 24 (18.8) | 13 (15.9) | |
| 6-week postpartum visit | 0.809 | |||
| Attended | 195 (92.9) | 119 (93.0) | 76 (92.7) | |
| Did not attend | 14 (6.7) | 9 (7.0) | 5 (6.1) | |
| Missing | 1 | -- | 1 (1.2) | |
| 12-week postpartum visit | 0.537 | |||
| Attended | 172 (85.2) | 107 (83.6) | 65 (79.3) | |
| Did not attend | 37 (17.6) | 21 (16.4) | 16 (19.5) | |
| Missing | 1 | -- | 1 (1.2) | |
| Infant survival | 0.120 | |||
| Alive | 179 (85.2) | 113 (88.3) | 66 (80.5) | |
| Dead | 31 (14.8) | 15 (11.7) | 16 (19.5) | |
| Infant HIV status | 0.113 | |||
| Positive | 3 (1.4) | 3 (2.3) | 0 (0.0) | |
| Negative | 126 (60.0) | 68 (53.1) | 58 (70.7) | |
| Missing | 81 | 57 (44.5) | 24 (29.3) | |
| Partner HIV status, n (%) | 0.079 | |||
| Positive | 68 (32.4) | 48 (37.5) | 20 (24.4) | |
| Negative | 73 (34.8) | 38 (29.7) | 35 (42.7) | |
| Unknown | 68 (32.4) | 41 (32.0) | 27 (32.9) | |
| Missing | 1 (0.5) | 1 (0.8) | -- | |
| Disclosure to partner | 0.903 | |||
| Yes | 188 (89.5) | 113 (88.3) | 75 (91.5) | |
| No | 12 (5.7) | 7 (5.5) | 5 (6.1) | |
| Missing | 10 (4.8) | 8 (6.3) | 2 (2.4) | |
| Partner involvement | 0.925 | |||
| Yes | 189 (90.0) | 115 (89.8) | 74 (90.2) | |
| No | 21 (10.0) | 13 (10.2) | 8 (9.8) | |
The 6- and 12-week postpartum visits were attended by the majority of participants (81.9% and 92.9%, respectively, Table 1). The majority of infants survived during the study period (85.2%) and were HIV-negative. Only 3 infants (1.4%) tested HIV-positive during the study period. An overwhelming majority (90%) of male partners attended an antenatal care visit on at least one occasion, and about 1 in 3 partners (32.4%, n=68) was HIV-positive. Only 12 participants (5.7%) acknowledged not disclosing their HIV status to their partner.
A greater proportion of adherent than non-adherent participants were of “Other” maternal ethnicity (37.5%, n=48 vs. 14.6%, n=12, P=0.006). There were no differences between adherent and non-adherent participants by maternal age, education, employment status, CD4+ cell count and postpartum clinic attendance. Infant HIV status, infant survival, partner HIV status, disclosure to partner and partner involvement in PMTCT did not vary by adherence status.
3.2. Main Variable (Dependent or Outcome) Results
Analysis with multivariate logistic regression (Table 2) showed that study arm, ethnicity, and partner HIV status were statistically significant predictors of maternal ART adherence. HIV-positive, ART-treated women in the intervention group were approximately 17 times more likely to adhere to their prescribed ART compared to women in the control group (adjusted odds ratio (aOR)=16.9, 95% CI: 5.30-54.24; p <0.001). Compared to women in the ethnic group designated as “Other”, Gwari women were 87% less likely to adhere to ART, and Nupe women were 66% less likely to adhere to treatment (P <0.002). Women having a partner with known HIV-positive status were approximately 3 times more likely to adhere to ART compared to women having a partner of unknown HIV status (aOR=3.14, 95%CI: 1.22-8.07).
| Variable | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Study arm | <0.001 | |
| Control | Ref | |
| Intervention | 16.95 (5.30-54.24) | |
| Maternal ethnicity | 0.002 | |
| Hausa-Fulani | 0.44 (0.17-1.14) | |
| Gwari | 0.13 (0.05-0.38) | |
| Nupe | 0.34 (0.14-0.87) | |
| Other | Ref | |
| Partner HIV status | 0.048 | |
| Positive | 3.14 (1.22-8.07) | |
| Negative | 1.50 (0.62-3.61) | |
| Unknown | Ref | |
OR=Odds Ratio; CI=Confidence interval; Ref=Referent category. Adjustment variables include: study arm, maternal ethnicity, and partner’s HIV status.
3.3. Other Variable (Covariates) Results
A total of 520 MASRI questionnaires were completed by the 210 participants. The number of questionnaires completed by each participant varied, and ranged from 1 to 10. More than 80% percent of the total survey responses indicated that there were no missed medication doses the previous day. A similar percentage of responses reported no missed doses in the past two days, three days, and two weeks. Almost half of responses indicated that participants never missed a single dose of prescribed ART, while 14% of responses reported a missed dose earlier in the week. The most commonly cited reason for non-adherence was concern regarding disclosure of HIV status (24.9%, 128 of 514 responses indicated “yes” to the question “I did not want others to know that I am taking my drugs,” (Figure 2). Being away from home was the second most common reason provided by respondents (14.1%, 73 of 516 responses).
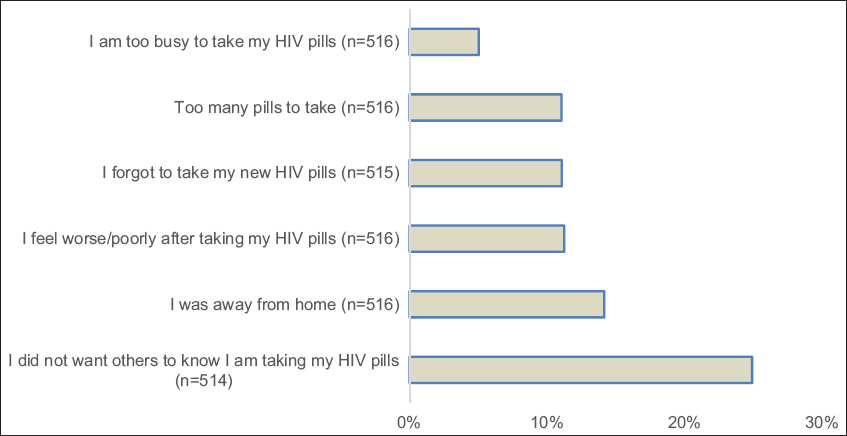
- Reasons for non-adherence to antiretroviral therapy among pregnant women, Niger state, northcentral Nigeria
4. Discussion
4.1. Discussion
We found that study arm, partner HIV status, and maternal ethnicity were independently associated with ART adherence in HIV-positive pregnant women in our study. Women who were enrolled in the intervention arm clinics had 17-fold higher odds of being adherent to ART compared to their female counterparts seen in the control arm sites. This finding is not surprising. Our trial intervention was comprised of task-shifting of PMTCT tasks to trained midwives, postpartum integration of mother-infant care, point-of-care CD4+ testing, and increased male partner engagement. We were unable to determine which of the specific components of the intervention was associated with adherence, but we could make some inferences. In our parent PMTCT trial total patient satisfaction rates were higher in the intervention arm compared to the control arm.27 Patient satisfaction may have increased the likelihood that a woman remained on ART once she initiates it.28 In addition, the male partner engagement component of the parent trial included specific community activities that targeted pregnant women and their partners.17,18 These activities could have impacted adherence through the increased level of engagement with the health system characterized by such opportunities.
Women of Gwari and Nupe ethnicity had a decreased likelihood of medication adherence compared to “Other” ethnic groups (comprised mostly of Igbo and Yoruba). This finding may be related to differences in literacy levels. The Igbo and Yoruba ethnic groups are located in the southern parts of Nigeria. Compared to ethnic groups in the north (Hausa-Fulani, Nupe, Gwari), groups in the south tend to have higher literacy rates.29 The association between literacy and adherence is well-documented.19,30 Basic literacy skills are required for health literacy and to understand and interpret health information. Therefore, limited literacy skills can negatively impact treatment adherence. The association between ethnicity and adherence could also be related to differences in cultural beliefs within ethnic groups, especially differences in beliefs surrounding effectiveness of ART and other treatment options (e.g., complementary and alternative medicine modalities), and about HIV itself. On an interpersonal and community level, stigma and discriminatory attitudes may be influenced by cultural beliefs and serve as a barrier to adherence.
Our finding that participants with HIV-positive partners had an increased likelihood of adherence is consistent with reports from India that found better adherence in seroconcordant vs. serodiscordant couples using plasma viral load as a proxy.31 Our result is, however, not consistent with Mitzel et al., who reported that couples in long-term serodiscordant relationships had better adherence than couples in long-term sero-concordant relationships.32
The most common reason for non-adherence reported by our respondents was related to concerns regarding disclosure of HIV status. Fear of HIV status disclosure is often related to stigma and discrimination, which are well-recognised barriers to adherence.28,33 To conceal her HIV status, a pregnant woman may choose not to seek HIV care, refuse treatment, or fail to take her ART appropriately. In those that do seek care, stigma and discrimination from health workers can be a barrier to retention in care, which in turn has a negative impact on adherence.
4.2. Limitations amd Strengths
Our study has limitations. First, we included responses from pregnant women who were enrolled in a major PMTCT trial in a specific region of Nigeria. Our findings may therefore not be generalizable to HIV-positive women from other parts of sub-Saharan Africa. Second, our definition of adherence was based on maternal self-report only, without correlation with biological assessments (e.g., viral load, a common proxy for adherence). At the time of this study, viral load testing was not yet standard-of-care in Nigeria. Third, the policy changes in PMTCT recommendations associated with Option B+ that advocate for ART initiation regardless of CD4+ cell count levels has made the use of CD4+ testing obsolete in decision-making regarding ART initiation in pregnancy. Nevertheless, the significance of having point-of-care laboratory testing is applicable to viral load testing, as the logistical challenges that previously confronted PMTCT programs regarding CD4+ testing equally likely apply to viral load testing in contemporary times. Finally, our use of secondary analysis limits our inquiry to only available data and variables. The strengths of our study included the use of a validated adherence questionnaire, the relatively large sample size, and the use of a rigorous statistical approach (multivariate analysis with adjustment for potential confounders) to identify independent factors associated with adherence.
4.3. Recommendation for Further Studies
Future studies should include considerations of biological assessments of adherence (e.g. viral load testing), account for the possible role of attrition associated with longitudinal follow up, and the contributions of recent policy changes to antiretroviral treatment in pregnancy (“Option B+”).
5. Conclusion and Global Health Implications
A comprehensive package of PMTCT services that includes task-shifting, point-of-care laboratory testing, male engagement and integrated maternal and newborn care had a positive impact on maternal adherence to ART, but overall adherence was low. The inclusion of some components of this service package in routine PMTCT programs (with viral load testing to substitute for CD4+ testing) might improve adherence in similar settings. While male partner engagement was associated with increased adherence, fear of disclosure remained a significant barrier to adherence. We recommend increased male partner involvement in antenatal care through partner HIV testing and counseling services and the use of peer mentors. Our findings also underscore the value of culturally-competent adherence counseling that address ethnocultural beliefs and practices and tailored according to the individual’s health literacy and education level. Finally, PMTCT programs should provide health care workers with training that employ stigma-reduction techniques and encourage respect for patient confidentiality.
Acknowledgements:
We thank the following FGH Nigeria staff: Dr. Usman Gebi, Dr. Mukhtar Muhammad, Dr. Saidu Ishaq, Mr. Awwal Gambo and Mr. Ibrahim Sodangi.
Conflicts of Interest: The authors declare no competing interests.
Funding/Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (1R01HD075075). The findings and conclusions here are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Ethics Approval: Ethics approval was obtained from the Nigeria National Human Research Ethics Committee and the Vanderbilt University IRB (IRB #121257). This adherence study received approval from the Institutional Review Board of Meharry Medical College (IRB #18-11-870).
References
- Lancet HIV. 2017;4(8):e349-e356. doi:10.1016/S2352-3018(17)30066-8
- Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe:comparisons with general population. Current Opinions in HIV AIDS. 2016;11(5):492-500.
- [Google Scholar]
- Immediate Antiretroviral Therapy Decreases Mortality Among Patients With High CD4 Counts in China:A Nationwide, Retrospective Cohort Study. Clinical Infectious Diseases. 2018;66(5):727-734. doi:10.1093/cid/cix878
- [Google Scholar]
- Life expectancy of HIV-positive people after starting combination antiretroviral therapy:a meta-analysis. HIV Medicine. 2017;18(4):256-266. doi:10.1111/hiv.12421
- [Google Scholar]
- Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. International Journal of Epidemiology. 2009;38(3):746-56. doi:10.1093/ije/dyp004
- [Google Scholar]
- Adherence to Antiretroviral Therapy During and After Pregnancy:Cohort Study on Women Receiving Care in Malawi's Option B+Program. Clinical Infectious Diseases. 2016;63(9):1227-1235.
- [Google Scholar]
- Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Medicine. 2008;5(5):e109. doi:10.1371/journal.pmed.0050109
- [Google Scholar]
- High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966-71. doi:10.1126/science.1228160
- [Google Scholar]
- Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clinical Infectious Diseases. 2003;37(8):1112-8.
- [Google Scholar]
- Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Annals of Internal Medicine. 2007;146(8):564-73.
- [Google Scholar]
- Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission Women and Infants Transmission Study Group. New England Journal of Medicine. 1999;341(6):394-402. PubMed PMID:10432324
- [Google Scholar]
- Low detectable postpartum viral load is associated with HIV transmission in Malawi's prevention of mother-to-child transmission programme. J ournal of the International AIDS Society. 2019;22(6):e25290. doi:10.1002/jia2.25290
- [Google Scholar]
- Loss to Follow-Up within the Prevention of Mother-to-Child Transmission Care Cascade in a Large ART Program in Nigeria. Current HIV Research. 2015;13(3):201-9.
- [Google Scholar]
- Elimination of Mother-To-Child Transmission of HIV Infection:The Drug Resource Enhancement against AIDS and Malnutrition Model. International Journal of Environmental Research and Public Health. 2015;12(10):13224-39. doi:10.3390/ijerph121013224
- [Google Scholar]
- National summary sheet preliminary findings. Published March 2019 https://www.naiis.ng/resource/factsheet/NAIIS%20PA%20NATIONAL%20FACTSHEET%20FINAL.pdf
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Published 2019 https://www.unaids.org/en/regionscountries/countries/nigeria
- Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria:a cluster-randomised controlled trial. Lancet HIV. 2016;3(5):e202-11. doi:10.1016/S2352-3018(16)00018-7 Epub 2016 Feb 24
- [Google Scholar]
- Optimizing PMTCT service delivery in rural North-Central Nigeria:protocol and design for a cluster randomized study. Contemporary Clinical Trials. 2013;36(1):187-97. doi:10.1016/j.cct.2013.06.013
- [Google Scholar]
- Post Option B+implementation programme in Nigeria:Determinants of adherence of antiretroviral therapy among pregnant women with HIV. International Journal of Infectious Diseases. 2019;81:225-230. doi:10.1016/j.ijid.2019.02.014
- [Google Scholar]
- Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J ournal of Biomedical Informatics. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010 10.21106/ijma.327
- [Google Scholar]
- Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269-277.
- [Google Scholar]
- Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials. 2004;5(2):74-79. doi:10.1310/JFXH-G3X2-EYM6-D6UG 10.21106/ijma.327
- [Google Scholar]
- A simple single-item rating scale to measure medication adherence:Further evidence for convergent validity. J ournal of the International Association of Providers of AIDS Care (Chicago). 2009;8(6):367-374. doi:10.1177/1545109709352884 10.21106/ijma.327
- [Google Scholar]
- Patterns of HIV care clinic attendance and adherence to antiretroviral therapy among pregnant and breastfeeding women living with HIV in the context of option B+in zimbabwe. J ournal of Acquired Immune Deficiency Syndromes. 2017;75(Suppl 2):S198-S206. doi:10.1097/QAI.0000000000001347 10.21106/ijma.327
- [Google Scholar]
- Correlation of adherence by pill count, self-report, MEMS and plasma drug levels to treatment response among women receiving ARV therapy for PMTCT in Kenya. AIDS and Behavior 2017 doi:10.1007/s10461-017-1724-7 10.21106/ijma.327
- [Google Scholar]
- Short-term effectiveness of a community health worker intervention for HIV-infected pregnant women in tanzania to improve treatment adherence and retention in care:A cluster-randomized trial. PLoS One. 2017;12(8):e0181919. doi:10.1371/journal.pone.0181919 10.21106/ijma.327
- [Google Scholar]
- Patient and Provider Satisfaction With a Comprehensive Strategy to Improve Prevention of Mother-to-Child HIV Transmission Services in Rural Nigeria. J ournal of Acquired Immune Deficiency Syndrome. 2016;72(Suppl 2):S117-23. doi:10.1097/QAI.0000000000001058
- [Google Scholar]
- A socio-ecological examination of treatment access, uptake and adherence issues encountered by HIV-positive women in rural north-central Nigeria. J ournal of Evidence-Based Social Work. 2018;15(1):38-51. doi:10.1080/23761407.2017.1397580
- [Google Scholar]
- 2010. Report of the National Literacy Survey. Available from http://www.nigerianstat.gov.ng/download/43
- Using a composite adherence tool to assess ART response and risk factors of poor adherence in pregnant and breastfeeding HIV-positive Cameroonian women at 6 and 12 months after initiating option B. BMC Pregnancy and Childbirth. 2018;18(1) 418-018-2058-9 doi:10.1186/s12884-018-2058-9
- [Google Scholar]
- Couples at risk for HIV infection in southern India:Characteristics of HIV-infected patients in concordant and discordant heterosexual relationships. International Journal of STD &AIDS. 2010;21(2):96-100. doi:10.1258/ijsa.2008.008418 10.21106/ijma.327
- [Google Scholar]
- The effect of partner serostatus and relationship duration on HIV medication adherence. AIDS and Behavior. 2019;23(2):499-503. doi:10.1007/s10461-018-2244-9
- [Google Scholar]
- The role of HIV stigma in ART adherence and quality of life among rural women living with HIV in India. AIDS and Behavior. 2018;22(12):3859-3868. doi:10.1007/s10461-018-2157-7
- [Google Scholar]





