Translate this page into:
Malaria, Helminth Infections and Clinical Status Among HIV-Infected Pregnant Women
*Corresponding author email: ademowo_g@yahoo.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background or Objectives:
Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome (HIV/AIDS) is widespread in sub-Saharan Africa with similarity in geographical distribution of major pathogens of public health interest. The aim of this study was to assess the effect of malaria and helminths on CD4 count, hematocrit values and viral load among HIV-infected pregnant women.
Methods:
One hundred and ninety-seven HIV-infected pregnant women aged 18-45 years were recruited from a registered HIV clinic and questionnaires were administered for socio-demographic details. Screening for malaria parasites in blood was through microscopy while helminths were identified in stool using Kato-Katz method. Hematocrit levels were determined through centrifugation of blood collected in capillary tubes. At the time of recruitment, most recent CD4 count and viral load was obtained from the patients’ case notes.
Results:
About three-quarters (73.6%) of the women had above primary school level of education while more than half (60.2%) were petty traders. The prevalence of malaria parasites in the blood samples was 24.9%, while 3% were infected with helminths. There was only a single case of malaria, helminths and HIV co-infection in the study group. Prevalence of anemia was 75.6% with eight cases (4.1%) of severe anemia. About 86.6% of the women with anemia had low CD4 count (χ2= 8.801, p=0.032). The mean CD4 count was significantly lower among those with co-infection of malaria and HIV.
Conclusion and Global Health Implications:
Malaria or helminth infection among HIV-infected women lowers the CD4 count and increases the viral load with little changes in hematocrit values. Routine screening of HIV-infected women for probable multiple infections will aid in improving their overall health and well-being.
Keywords
HIV
Co-infection
CD4 count
Anemia
Pregnancy
Malaria
Nigeria
1. Introduction
1.1. Background of the Study
HIV/AIDS is a disease of major public health importance on the global scale. An estimated 36.9 million people were living with HIV in 2017 with 1.8 million new infections.1 It remains a global menace with a high record of 35.4 million HIV-related deaths since the onset of the epidemic and in 2017 alone, 940,000 people died from AIDS-related illnesses.1 Presently, about 3,229,757 people are living with HIV in Nigeria with the high risk groups being the sexually active, those on hard drugs and transgender women.2 HIV overlaps with other infectious diseases in its distribution across sub-Saharan Africa thus raising the possibility of co-infections.3,4
Pregnant women, being a sexually active group are at a higher risk of HIV and are more susceptible to malaria and helminths particularly due to the lowered immunity in pregnancy.5 In 2017, there were about 219 million cases of malaria with a quarter of this global figure occurring in Nigeria.6 Malaria during pregnancy among HIV-infected mothers has been attributed to higher parasite densities, severe anemia, febrile illnesses and adverse birth outcomes.7,8 The burden of helminthiasis is likewise felt globally with an estimated one billion people infected annually.9 Some factors identified as risks for helminthiasis among HIV-infected pregnant women were CD4 count ≤350 cells/mm3 and detectable viral load.10
In Nigeria, some studies have established higher prevalence and risk of malaria among HIV-seropositive pregnant women.8,11,12 Some others reported anaemia and low CD4 count among those with malaria and HIV co-infection.12–15 Another study reported higher viral load count.15 However, no study has evaluated the effect of helminths alongside malaria in the HIV population of pregnant women. This study was conducted to determine the prevalence of malaria and helminths among HIV infected pregnant women and effect on maternal CD4 counts, viral load and anemia.
2. Methods
2.1. Study Variables
Study design
The study was carried out within Ibadan metropolis, a city in Southwest Nigeria as a cross-sectional survey. One hundred and ninety-seven pregnant women aged 18-45 years were recruited from the HIV clinic of a secondary health care facility in Ibadan, Southwest Nigeria. Informed consent was obtained from all the participants and they were screened for presence of malaria parasites in blood and helminths in stool. The most recent CD4 count and viral load at the time of recruitment were obtained from the HIV-infected patients’ case notes. Women with obvious complications in pregnancy and those who did not provide both stool and blood samples were excluded from the study. Questionnaires were administered for personal details, socio-demographic details and use of antiretroviral, antihelminthics and antimalarial drugs by trained data collectors in the native language of the women (Yoruba) as majority of them were semi-literate.
Laboratory tests
Giemsa stained thick blood smears were screened under a light microscope at x1000 magnification for malaria parasites.16 Stool samples were examined microscopically for helminths using Kato-Katz method.17 The stool count was multiplied by 24 to obtain the eggs load per gram of stool. Hematocrit values were obtained by withdrawing venous blood into heparinized capillary tube and sealed at one end with plasticine. The tubes were spun at 3000 rpm for 10 mins in a microhematocrit centrifuge (Hawksley Ltd, Lancing, UK) and read with Hawksley hematocrit reader. In pregnancy, hematocrit levels less than 33% is an indication of anemia.18 The hematocrit values were further classified as normal (≥33%), mild anemia (30-32%), moderate anemia (21-29%) and severe anemia (<21%).
2.2. Statistical Analysis
Descriptive statistics were used to summarize quantitative variables while qualitative variables were summarized with proportions. Frequency tables and graphs were used to present relevant variables. The association between variables were analysed using Pearson Chi-square or Fisher Exact Test where applicable. Student’s t-test and ANOVA were used to test for significant differences (p<0.05). Ethical approval for this study was obtained from the Joint University of Ibadan/University College Hospital Ethical Review Committee and the Oyo State Ministry of Health Ethical Review Committee.
3. Results
3.1. Sociodemographic Characteristics
The average age of the women was 30.68±4.96 years. Majority (92.1%) of the women were multigravidae and 58.1% were in the third trimester. About three-quarters (73.6%) of the women had secondary or post-secondary school education while more than half (60.2%) were petty traders (Table 1).
| Parameters | n | Frequency (%) |
|---|---|---|
| Age | 195 | |
| <20 years | 1 (0.5%) | |
| 20-34 years | 147 (75.4%) | |
| ≥35 years | 47 (24.1%) | |
| Gestational period | 172 | |
| First Trimester | 7 (4.1%) | |
| Second Trimester | 65 (37.8%) | |
| Third Trimester | 100 (58.1%) | |
| Gravidity | 190 | |
| Primigravidae | 15 (7.9%) | |
| Multigravidae | 175 (92.1%) | |
| Level of Education | 197 | |
| None/Primary/Quranic | 52 (26.4%) | |
| Secondary/Post-secondary | 145 (73.6%) | |
| Occupation | 196 | |
| Student/unemployed | 20 (10.2%) | |
| Petty traders | 118 (60.2%) | |
| Business/Professionals | 58 (29.5%) | |
3.2. Infection Status
Forty-eight women (24.4%) had co-infection of malaria and HIV while five women (2.5%) had co-infection of helminth and HIV. Only one (0.5%) of the HIV-infected women had co-infection of both malaria and helminths. Ascaris lumbricoides was the only helminth species present.
3.3. Infection Status and Host Parameters
There was 75.6% (149/197) prevalence of anemia among the women. Eight (4.1%) of them had severe anemia (PCV< 21%) while majority (43.1%) had moderate anemia. The lowest hematocrit value (27%) was in the HIV patient with co-infection of malaria and helminths while those with helminth and HIV co-infection had the highest mean value (29.25±3.10%) but these values were not statistically significant across the groups (p=0.820).
CD4 count and viral load values were obtained for 82 out of 197 pregnant women. The CD4 count was grouped as low CD4 (<350 cells/mm3) and high CD4 (≥350 cells/mm3). Regardless of infection status, a considerable proportion of these women (86.6%) were anemic while 45.1% (37/82) had low CD4 count with significant association between the severity of anemia and level of CD4 count (χ2= 8.801, p=0.032). The association in CD4 count and anemia remained significant among those infected with HIV only (χ2= 8.571, p=0.036). As shown in Table 2, there was no significant association between infection status and CD4 count for all the infection groups but the association between presence of anemia and HIV infection only was highly significant (χ2= 7.378, p=0.007).
| Infection status | CD4 count status | n | x2, P-value | Anemia absent | Anemia present | x2, P-value | Total |
|---|---|---|---|---|---|---|---|
| HIV only | Low CD4 | 26 | 1.629, 0.202 | 0 | 26 (100%) | 7.378, 0.007 | 63 |
| High CD4 | 37 | 9 (24.3%) | 28 (75.7%) | ||||
| Mal/HIV | Low CD4 | 9 | 1.641, 0.200 | 1 (11.1%) | 8 (88.9%) | 0.714, 0.398 | 15 |
| High CD4 | 6 | 0 | 6 (100%) | ||||
| Hel/HIV | Low CD4 | 1 | 0.175, 0.676 | 0 | 1 (100%) | 0.750, 0.386 | 3 |
| High CD4 | 2 | 1 (50%) | 1 (50%) | ||||
| Mal/Hel/HIV | Low CD4 | 1 | 1.231, 0.267 | 0 | 1 (100%) | - | 1 |
| High CD4 | 0 | 0 | 0 | ||||
| Total | 82 | 3.103, 0.376 | 11 (13.4%) | 71 (86.6%) | 6.661, 0.010 | 82 | |
Mal – Malaria, Hel – Helminths, HIV – Human Immunodeficiency Virus
The mean CD4 count was significantly higher among those infected with HIV only compared with those with co-infection of malaria and HIV. However, the viral load and hematocrit values were higher in those with co-infection of malaria and HIV but these were not statistically significant (Table 3). There was no significant difference in CD4 count, viral load and hematocrit values of those with co-infection of helminths and HIV and those with HIV only (Table 3).
| Parameters | Mean count±SEM | ||
|---|---|---|---|
| CD4 count (cells/mm3) | Viral load (copies/mL) | Hematocrit (%) | |
| Malaria/HIV | 319.27±40.49 | 228893.73±102872.13 | 29.73±0.70 |
| HIV only | 447.79±24.57 | 57833.79±10143.64 | 29.30±0.42 |
| n | 78 | 78 | 191 |
| P value | 0.020 | 0.120 | 0.605 |
| Helminth/HIV | 426±68.94 | 73504.67±62247.42 | 30.60±1.81 |
| HIV only | 447.79±24.57 | 57833.79±10143.64 | 29.30±0.42 |
| n | 66 | 66 | 148 |
| P value | 0.849 | 0.746 | 0.566 |
3.4. Correlations of Host Parameters and Infection Status
As shown in Figure 1, there was a negative linear relationship between viral load and CD4 count among those with co-infection of malaria and HIV (r = -0.812, p<0.0001) and those with HIV only (r = -0.564, p<0.0001). However, there was positive correlation between gestational age and viral load among those with co-infection of malaria and HIV (r = 0.522, p=0.046). Correlations of host parameters in those with helminth and HIV co-infection were excluded from analysis due to the small number of samples.
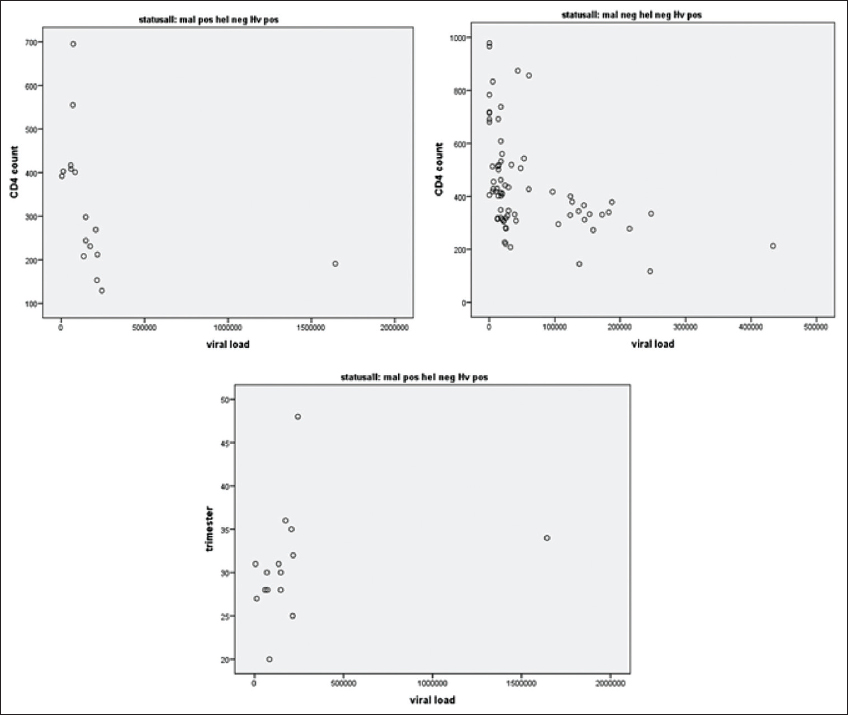
- Correlations in CD4 count, viral load and gestational age among those infected with HIV only and co-infection of malaria and HIV
4. Discussion
The prevalence of malaria, helminths and their co-infections was determined among HIV infected pregnant women reporting for follow-up clinic in Ibadan, Southwest Nigeria. Prevalence of malaria was 24.9% while that of helminth infection was 3%. It has been established that pregnant women are more susceptible to malaria but they are usually asymptomatic.10,19 A recent review article reported the prevalence of malaria and HIV co-infection among pregnant women in sub-Saharan Africa as 0.94% - 37%.20 The prevalence reported in this study is within that range. Similarly, 33.3% and 21% prevalence of malaria and HIV co-infection has been reported in Nigeria and East Africa respectively.13,21
The only helminth species found in this study was Ascaris lumbricoides. This helminth has been reported as the predominant of the helminth species in various studies carried out in Nigeria.22–24 Furthermore, the prevalence of helminths (3.0%) in this study was very low compared with 34.3% prevalence reported in a similar study conducted in East Africa.10 The likely reason for the difference in result could be due to the difference in study population. This study was carried out in a secondary health care facility in an urban setting while the Rwanda study was done in health centers in rural and peri-urban locations.
Prevalence of anemia among the pregnant women in this study was 75.6%. In a previous study, HIV has been identified as a significant risk factor for anemia.25 In this study, the mean hematocrit values was lowest in malaria, helminths and HIV co-infections but the test of significance could not be ascertained since there was only a single patient with that status. Multiple infections are expected to further predispose to anemia as chronic diseases such as malaria, tuberculosis, HIV and diabetes often worsen anaemic conditions.26 Furthermore, a greater percentage (62.4%) of the anaemic women had moderate or severe anemia. A similar finding was reported among HIV-infected women in a previous study.27
Interestingly, those with co-infection of helminths and HIV had higher hematocrit values relative to those with HIV only howbeit insignificant. Predisposition to anemia is multifactorial18 but there could be the possibility of a protective role of helminths against HIV-induced anemia. However, this can only be substantiated in large cohort studies.
In this study, a significantly higher proportion of those with low CD4 count were anaemic. CD4 count is a known risk factor for anemia.28 The CD4 count was significantly lower among those with malaria and HIV co-infection relative to those with HIV only. Low CD4 count in malaria and HIV co-infection was earlier reported in Nigeria.29 This may increase the risk of HIV disease progression and mother-to-child transmission of HIV.30 Hence, prompt screening and adequate treatment of malaria should be encouraged in pregnancy particularly among HIV-infected women. Similarly, it was observed that those with co-infection of helminth and HIV had a lower CD4 count but this was not significant relative to those with HIV only.
The mean viral load and hematocrit values were not significantly different in the co-infection groups relative to those with HIV-infection only. Likewise, the proportion of those with low CD4 count and low hematocrit values among those with co-infection relative to HIV only was not significant. This insignificant finding in haematological parameters was also reported in a study where 81% of the women were on antiretroviral drugs and only six out of the sixteen haematological parameters tested were significant between the groups. 29 One of the limitations of this study is that there were few cases of co-infection particularly helminth and HIV co-infection which prevented a robust statistical analysis. The inaccessibility of the CD4 count of all the recruited women is also a limitation.
5. Conclusion and Global Health Implications
Co-infection of malaria and HIV is relatively high in the study population and this adversely affected the CD4 count and viral load. This implies a worsening of HIV prognosis in this population. Improved clinical management of HIV patients with pregnancy would be achieved through routine diagnosis of malaria and helminths. In addition, intermittent preventive treatment of malaria in pregnancy (IPTp) in endemic areas should be implemented.
Acknowledgements:
The participants, field and laboratory staff are duly acknowledged for their participation in the study.
Compliance with Ethical Standards
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Financial Disclosure: The authors have no financial disclosures to discuss.
Funding/Support: This work is part of a larger project funded by European Community’s Seventh Framework Programme (HEALTH-F3-2009-241642) and also supported by the Institute of Infectious Diseases of Poverty (IIDP) Scholarship Award ID Number 2013/08 from 2013 to 2016 awarded to ORR. None of the funding sources were involved in study design, collection, analysis and interpretation of the data, in the writing of the paper or in the decision to submit the paper for publication.
Ethics Approval: The Joint University of Ibadan/University College Hospital Ethical Review Committee (UI/EC/10/0180) and the Oyo State Ministry of Health Ethical Review Committee approved this study.
Disclaimer: None.
References
- 2018. Global HIV Statistics. http: //www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- HIV epidemiology in. Saudi J Biol Sci. 2018;25(4):697-703. doi:10.1016/J.SJBS.2016.03.006
- [Google Scholar]
- Tropical Anemia:One of Africa's Great Killers and a Rationale for Linking Malaria and Neglected Tropical Disease Control to Achieve a Common Goal. PLoS Negl Trop Dis. 2008;2(7):e270. doi:10.1371/JOURNAL.PNTD.0000270
- [Google Scholar]
- Spatial overlaps in the distribution of HIV/AIDS and malaria in Zimbabwe. BMC Infect Dis. 2018;18(1):598. doi:10.1186/s12879-018-3513-y
- [Google Scholar]
- Adjuvanted Vaccines in Pregnancy: What is Known About Their Safety? Expert Rev Vaccines. 2010;9(12):1411-1422.
- [Google Scholar]
- 2018. World Malaria Report. https: //www.who.int/malaria/publications/world-malaria-report-2018/en/ www.who.int/malaria
- The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71(Suppl 2):41-54.
- [Google Scholar]
- Comparative Analysis of Asymptomatic Malaria Parasitaemia in Human Immunodeficiency Virus Positive and Negative Pregnant Women in Sagamu, Ogun State, South West Nigeria. IOSR J Dent Med Sci. 2018;17:59-65. doi:10.9790/0853-1704155965
- [Google Scholar]
- Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7(1):37-55. doi:10.1186/1756-3305-7-37
- [Google Scholar]
- Helminthic Infections Rates and Malaria in HIV-Infected Pregnant Women on Anti-Retroviral Therapy in Rwanda. PLoS Negl Trop Dis. 2013;7(8):1-9. doi:10.1371/journal.pntd.0002380
- [Google Scholar]
- Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria. J Vector Borne Dis. 2007;44:250-254.
- [Google Scholar]
- Malaria and HIV co-infection and their effect on haemoglobin levels from three healthcare institutions in Lagos, Southwest Nigeria. Afr Health Sci. 2013;13(2):295-300. doi:10.4314/ahs.v13i2.14
- [Google Scholar]
- Malarial Infection in HIV Infected Pregnant Women Attending a Rural Antenatal Clinic in Nigeria. 2014. Adv Epidemiol. 2014 http: //dx.doi.org/10.1155/2014/694213
- [Google Scholar]
- Effect of Malaria on Cellular Immunity of Pregnant Women Coinfected with Effect of Malaria on Cellular Immunity of Pregnant Women Coinfected with Malaria and HIV in Sokoto State, North-Western Nigeria. Int J Clin Med Res. 2018;5(3):61.:66.
- [Google Scholar]
- The epidemiology of HIV seropositive malaria infected pregnant women in Akure Metropolis, Southwestern Nigeria. Ann Trop Med Public Heal. 2013;6(5):519. doi:10.4103/1755-6783.133703
- [Google Scholar]
- Bench Aids for the Diagnosis of Intestinal Parasites. (original version 1994, corrected in 2012). https: //www.who.int/neglected_diseases/resources/ 9789241544764/en/
- 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. https: //www.who.int/vmnis/indicators/haemoglobin/en/
- Prevalence of malaria at booking among antenatal clients in a secondary health care facility in Ibadan, Nigeria. Afr J Reprod Health. 2008;12(2):141.:152.
- [Google Scholar]
- Malaria and HIV coinfection in sub-Saharan Africa:prevalence, impact, and treatment strategies. Res Rep Trop Med. 2018;9:123-136. doi:10.2147/RRTM.S154501
- [Google Scholar]
- Malaria and helminthic co-infection among HIV-positive pregnant women :Prevalence and effects of antiretroviral therapy. Acta Trop. 2012;124(3):179-184. doi:10.1016/j.actatropica.2012.08.004
- [Google Scholar]
- Soil-transmitted helminth infections in Nigerian children aged 0–25 months. J Helminthol. 2009;83(03):261. doi:10.1017/S0022149X08201252
- [Google Scholar]
- Prevalence and pattern of soil-transmitted helminthiasis among pregnant women in a tertiary health facility, southeast Nigeria. African J Med Heal Sci. 2014;13(1):56. doi:10.4103/2384-5589.139445
- [Google Scholar]
- Co-infection of malaria and intestinal parasites among pregnant women in Edo State, Nigeria. J Med Trop. 2017;19(1):43. doi:10.4103/jomt.jomt_42_16
- [Google Scholar]
- Prevalence of HIV and anemia among pregnant women. N Am J Med Sci. 2011;3(12):548-551. doi:10.4297/najms.2011.3548
- [Google Scholar]
- Anemia and pregnancy:A link to maternal chronic diseases. Int J Gynecol Obstet. 2011;115:S11-S15. doi:10.1016/S0020-7292(11)60005-2
- [Google Scholar]
- Risk of anaemia in HIV positive pregnant women in Ibadan, south west Nigeria. Afr J Med Med Sci. 2011;40(1):67-73.
- [Google Scholar]
- Anaemia in pregnancy is associated with advanced HIV disease. PLoS One. 2014;9(9):e106103. doi:10.1371/journal.pone.0106103
- [Google Scholar]
- Effect of HIV and malaria parasites co-infection on immune-hematological profiles among patients attending anti-retroviral treatment (ART) clinic in Infectious Disease Hospital Kano, Nigeria. PLoS One. 2017;12(3):e0174233. doi:10.1371/journal.pone.0174233
- [Google Scholar]
- HIV and malaria interactions:where do we stand? Expert Rev Anti Infect Ther. 2012;10(2):153-165. doi:10.1586/eri.11.167
- [Google Scholar]





