Translate this page into:
Nutritional Deficiencies and Food Insecurity Among HIV-infected Children in Tanzania
✉Corresponding author email: fvr@dartmouth.edu.
-
Received: ,
Accepted: ,
Abstract
Background:
Poor nutrition has been associated with impaired immunity and accelerated disease progression in HIV- infected children. The aim of this study was to quantify the levels of nutrient intake in HIV-infected children and compare these to standard recommendations.
Methods:
We surveyed HIV-infected Tanzanian children enrolled in a pediatric care program that provided routine nutritional counseling and vitamin supplementation. We obtained anthropometric measurements and determined 24-hour macronutrient and micronutrient intakes and food insecurity. Values were compared to recommended nutrient intakes based on age and gender.
Results:
We interviewed 48 pairs of children and their caregiver(s). The age of the child ranged from 2-14 years; median age 6 and 60% female. The median weight-for-height z-score for children ≤ 5 years was 0.69 and BMI-for-age z-scores for children >5 was -0.84. Macronutrient evaluation showed that 29 (60%) children were deficient in dietary intake of energy; deficiency was more common in older children (p=0.004). Micronutrient evaluation shows that over half of study subjects were deficient in dietary intake of vitamin A, vitamin D, vitamin E, thiamine, riboflavin, niacin, folate, vitamin B12, and calcium. Food insecurity was reported by 20 (58%) caregivers.
Conclusions and Public Health Implications:
The diets of many HIV-infected children at a specialized treatment center in Tanzania do not meet recommended levels of macro- and micro-nutrients. Food insecurity was a contributory factor. Enhanced dietary counseling and provision of macro- and micro-nutrient supplements will be necessary to achieve optimal nutrition for most HIV-infected children in resource-poor regions.
Keywords
HIV
Tanzania
Children
Energy Intake
Food Insecurity
Micronutrients
Introduction
The HIV epidemic has severely impacted the health and nutrition of children living in areas highly affect-ed by the disease. In Tanzania alone, an estimated 160,000 children were living with HIV/AIDS in 2009.[1,2] Tanzania has high rates of pediatric mal-nutrition due to beliefs about nutritional needs and preferred foods for children, limited health servic-es,[3] and low socioeconomic status.[4]
The combination of malnutrition and HIV introduc-es a circular attack on immune system function. HIV infection has been associated with increased physical and developmental growth requirements, common-ly resulting in symptomatic weight loss.[5,6] Infection requires increased caloric and protein requirements to fuel viral production and maintain cellular immune response[7]. Malabsorption of fats[8] and loss of nu-trients[9] has been observed during all stages of HIV infection. Poor nutrition has been shown to impair immune response, [10] accelerating the progression of disease, and can even serve as a mortality predictor in pediatric HIV cases.[11] The continuous cycle of mal-nutrition and HIV severity indicates that initiation of antiretroviral therapy (ART) is not the only important factor in treating HIV; meeting proper nutritional needs is an essential requirement as well.
Previous studies from sub-Saharan Africa have examined dietary intake or nutritional status among selected HIV-infected or HIV-exposed infants[12,13] or children with parasitic intestinal infections[14] with severe or moderate malnutrition. However, we are not aware of previous studies that have attempted to quantify dietary intake of macro-and micro-nutrients among an unselected cohort of HIV infected children in sub-Saharan Africa across a broad pediatric age spectrum. This study is a cross-sectional design that assessed nutritional deficiencies among HIV-infected children age 2-14 at different stages of HIV infection at a pediatric care program in Tanzania.
Methods
Study Population. The DarDar Pediatric Program (DPP) is a pediatric HIV care and treatment center in Dar es Salaam jointly operated by Geisel School of Medicine at Dartmouth and Muhimbili University of Health and Allied Sciences (MUHAS). Routine nutritional counseling is provided by trained nurse coun-selors, and children with moderate to severe malnu-trition as defined by the World Health Organization (WHO)[15] are seen by an in-house nutritionist for further counseling. Children are provided with daily multivitamins containing 1 mg thiamine HCl, 0.5 mg pyridoxine HCl BP, 1 mg riboflavin BP 1 mg, 15 mg ascorbic acid BP, 7.5 mg nicotinamide BP, and 1 mg cal-D-penthothenate BP. Clinic policy specifies provision of plumpy'nut, a micronutrient fortified ready-to-use therapeutic food for children meeting WHO guidelines for severe malnutrition.
Study subjects were current patients of the clinic between the ages of 2 and 14 years. Subjects were selected by convenience sampling for an equal distribution across parameters of age and HIV severity. To ensure representation of the entire patient population, subjects were stratified by age into two groups (2-6 years and 7-14 years) and by their most recent CD4 percentage obtained within the last 6 months (>25%, 16%-25%, ≤15%) as recorded in their patient file with the DPP. Data were collected to obtain a sample size of approximately eight subjects per age and CD4 group.
Data Collection. Subjects were recruited, enrolled, and interviewed during a previously scheduled visit to the DPP clinic, requiring them to stay for an additional hour. Interviews were conducted in Kiswahili by a clinic nurse or nutrition staff member trained by the study team. All interviews were conducted with the child subject and their caregiver (parent, relative, other caregiver) and included a medical history and information to determine household food insecurity. A trained nurse measured the subject's height, weight, and triceps skinfold-thickness using calibrated instruments regularly used by the clinic staff.
Dietary Assessment. Dietary intake was evaluated using a multiple pass 24-hour recall to list and quant ify all beverages and foods consumed in the previous day.[16] Energy, protein and micronutrient intakes were then calculated utilizing the Tanzania Food Composition Tables[17].
We compared estimated intakes to recomm-endations of the WHO for energy,[18] protein[19] and micronutrients,[20] which are age-and gen-der-specific. As per WHO guidelines, daily energy intake was increased an additional 10% for indi-viduals with HIV.[21] Due to a lack of standardized recommendations for several macr onutrients in resource-poor countries, children's diets were analyzed by percentage of total calories coming from each of the four macronutrient sources: fat, saturated fat, protein, and carbohydrates.
Food Insecurity. All subject caregivers were admin-istered the Household Food Insecurity Access Scale (HFIAS), which was adapted from the U.S. Agency for International Development (USAID) Food and Nutritional Technical Assistance II Project (FAN-TA-2) to determine availability and accessibility of food in each caregiver's household.[22] An adapted questionnaire contained a total of nine yes or no questions followed by a “frequency-of-occurrence” question. Some questions inquired about the subject caregiver's perception of food vulnerability while other questions addressed the caregiver's behavio-ral responses due to food insecurity. Based on the answers given by the caregiver a score was assigned to each subject ranging from 0 (no insecurity) to 27 (maximum insecurity) and the prevalence of food in-security for the sample population was determined. Food insecurity prevalence was calculated using previously established guidelines[22] that categorize households as food secure, or mildly, moderately or severely food insecure.
Data Analysis. All data were transcribed from the study forms into a password-protected Excel 2008 spreadsheet. Z-scores were calculated using the Nutristat software program in Epi Info version 7.I.0.6 (U.S. Centers for Disease Control and Pre-vention, Atlanta, GA.). The daily intake of macro-nutrients and micronutrients were compared to the recommended values for each of the study subjects based on the subject's age and gender. Statistical analyses were conducted using the computer pro-gram R version 2.15.1 (Vienna, Austria). Food inse-curity scores and categories for each subject were calculated as previously described by USAID FAN-TA-2.[22]
Ethical Statement. This study was conducted according to the guidelines laid down by the Declaration of Helsinki and all procedures involving human subjects were approved by the Committee for the Protection of Human Subjects at Dartmouth College and the Research Ethics Committee at Muhimbili University of Health and Allied Sciences (MUHAS) in Dar es Saalam, Tanzania. Written informed assent was obtained from all study participants aged nine years or older and written informed consent from a caregiver was obtained for study participants under the age of nine years.
Results
Subjects. Forty-eight children were enrolled (Table 1): 29 (60%) female; median age 6 years; median weight-for-height z-score for children ≤5,0.69; and median BMI-for-age z-score for children >5, -0.84. Forty-one (85%) children were on antiretroviral therapy (ART), with the most frequent regimens being azidothymidine (AZT)/lamivudine (3TC)/nevirapine (NVP) in 24 subjects (59%) and lamivudine (3TC)/nevirapine (NVP)/stavudine (d4T) in 14 children (34%).
| Characteristics | Participants, no. (%) |
|---|---|
| Age, median (range), years | 6(2-14) |
| Age Category, years | |
| 2-6 | 23 (48%) |
| 7-14 | 25 (52%) |
| Sex | |
| Female | 29 (60%) |
| Male | 19 (40%) |
| Height-for-Age z-scores, median (range) | -1.28 (-5.29-1.45) |
| < -2 and > -3 SD (moderate stunting) | 10 (21%) |
| < -3 SD (severe stunting) | 8 (17%) |
| Weight-for-Age z-scores, median (range) | -1.28 (-4.94-1.6) |
| < -2 and > -3 SD (moderate underweight) | 5 (10%) |
| < -3 SD (severe underweight) | 8 (17%) |
| Weight-for-Height z-scores (age≤5), median (range) | 0.69 (-1.28-1.51) |
| < -2 and ≥ -3 SD (moderate wasting) | 0 (0%) |
| < -3 SD (severe wasting) | 0 (0%) |
| BMI-for-Age z-scores (age>5), median (range) | -0.84 (-2.45-1.55) |
| < -2 and ≥ -3 SD (moderate wasting) | 3 (6%) |
| < -3 SD (severe wasting) | 0 (0%) |
| CD4% category | |
| ≤15% | 15 (31%) |
| 16% - 25% | 16 (33%) |
| > 25% | 17 (35%) |
| ARTa Treatment | 41 (85%) |
aART antiretroviral therapy
Anthropometrics. Median height-for-age, weight-for-age, and weight-for-height/BMI-for-age z-score values were -1.07, -1.23, and 0.5 for children age 2-6 and -2.02, -1.39, and -1.02 for children age 7-14, respectively. For children age 2-6,5 (22%) classified at stunted (low height-for-age z-scores), with 2 (9%) at the moderate level and 3 (13%) were severely stunted. Four children (17%) classified as underweight (low weight-for-age z-scores), with 3 (13%) at the moderate level and I (4%) was severely underweight. There was l child (4%), age 6, with a moderate level of wasting (low BMI-for-age z-score). For children age 7-14,13 (52%) classified at stunted, with 8 (32%) at the moderate level and 5 (20%) were severely stunted. Nine children (36%) classified as underweight, with 2 (8%) at the moderate level and 7 (28%) were severely underweight. There were 2 children (8%) in this age range with a moderate level of wasting.
Macronutrients. 29 children (60%) had dietary energy intakes that did not meet WHO recom-mendations for HIV-infected children (Table 2). Only 3 children were recorded as having been prescribed plumpy'Nut though none reported consumption on the 24-hour food recall conducted for this study. Deficient energy intake was more common in older children and in children with the lowest CD4 percentages (Figure 1). Anthropometric z-scores were lower for children that did not meet WHO energy recommendations compared to those who did meet recommendations: mean height-for-age (-1.85 vs -1.12, p-value 0.080), weight-for-age (-1.90 vs -0.84, p-value 0.004) and BMI-for-age (-0.90 vs -0.02, p-value 0.006). Only 1 child, age 5, (2%) failed to meet WHO recommendations for protein intake.
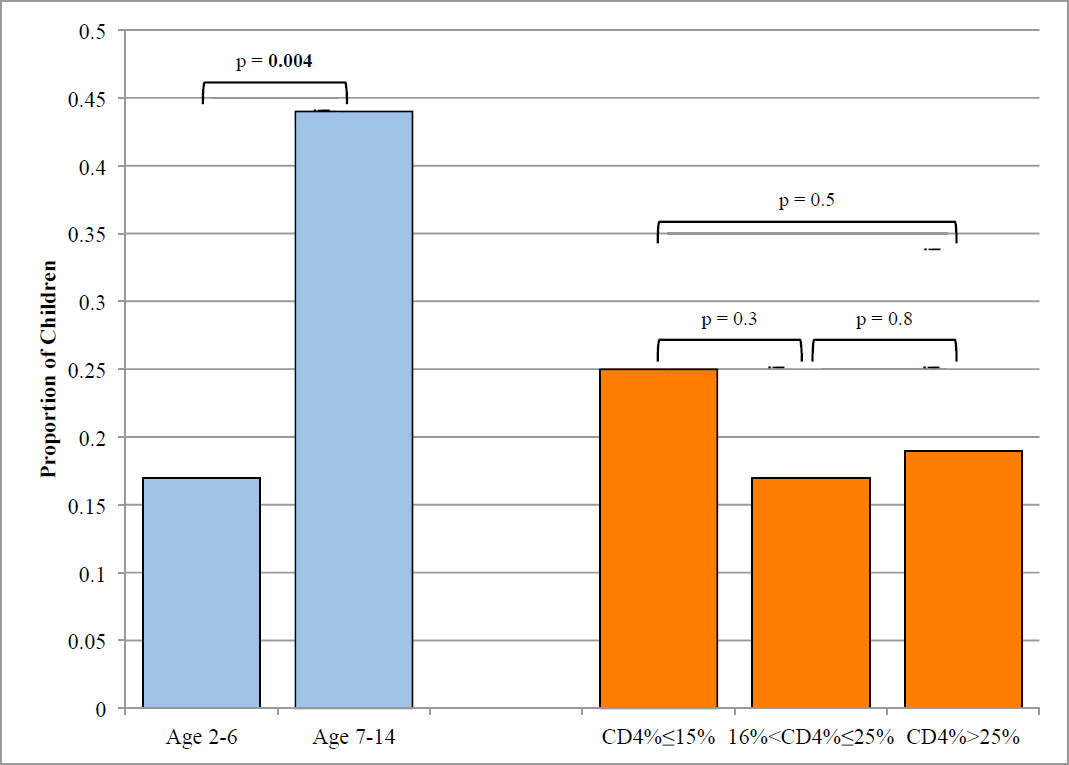
- Proportion of children who did not meet energy recommendations by age and CD4%
| Micronutrient | Median (Range) | Did not meet recommended values from diet | Did not meet recommended values from diet plus vitamin supplement |
|---|---|---|---|
| Vitamin A (Mg RAE) | 330 (0 - 8758) | 31 (65%) | 31 (65%) |
| Vitamin D (Mg) | 0 (0 - 3) | 48 (100%) | 48 (100%) |
| Vitamin E (mg) | 4 (0 - 13) | 37 (77%) | 37 (77%) |
| Vitamin C (mg) | 67 (1 - 213) | 10 (21%) | 8 (17%) |
| Thiamine (mg) | 0.6 (0.1 - 1.2) | 37 (77%) | 0 (0%) |
| Riboflavin (mg) | 0.5 (0 - 18) | 38 (79%) | 0 (0%) |
| Niacin (mg) | 8 (3 - 15) | 32 (67%) | 32 (67%) |
| Vitamin B6 (mg) | 1.1 (0.5 - 20) | 5 (10%) | 0 (0%) |
| Folate (µg) | 203 (79 - 535) | 32 (67%) | 32 (67%) |
| Vitamin B12 (Mg) | 0.5 (0 - 46) | 36 (75%) | 36 (75%) |
| Calcium (mg) | 255 (29 - 1303) | 46 (96%) | 46 (96%) |
| Iron (mg) | 8 (2-16) | 17 (35%) | 17 (35%) |
| Zinc (mg) | 5 (1 - 9) | 25 (52%) | 25 (52%) |
An average of 32% of the children's caloric intake came from fat and 20% came from saturated fat. Eight percent of calories came from protein and 60% from carbohydrates. These percentages were similar between the two age groups with the exception of saturated fat, which constituted a higher proportion of caloric intake in children age 7-14 than children age 2-6 (25% vs 16%, p-value = 0.0002).
Micronutrients. Micronutrient intakes from diet alone and from diet plus the multivitamin supplement are shown in Table 2. Although a total of 38 (79%) children reported receiving the multivitamin supplement, clinic staff reported that compliance was often poor especially among older children. Based on dietary sources alone, greater than 50% of the children had intakes that were deficient in the following micronutrients: vitamin A, vitamin D, vitamin E, niacin, folate, vitamin B12, calcium, and zinc; over 95% of children had deficient dietary intake of vitamin D and calcium. Assuming all 38 children were taking the multivitamin, 8 (21%) remained deficient in vitamin C intake, but none remained deficient in thiamine, riboflavin, or vitamin B6.
Children with a height-for-age z-score below or equal to -2 or weight-for age-scores below -2 had lower reported dietary vitamin D intake than those with a score above -2 (p=0.04 for both comparisons). Children with height-for-age z-scores below -3 had lower intakes of vitamin E and calcium than subjects whose z-scores were above -3 (p-values both 0.01).
Children with weight-for-age z-scores below or equal to -3 had lower reported vitamin E intake than those with a score above -3 (p-value = 0.007).
Food Insecurity. The median Food Insecurity score was 1 (range 0 to 11), with 39 (81%) of caregivers responding affirmatively to one or more questions regarding anxiety and uncertainty about household food income and 18 (38%) reporting some form of insufficient food quality and intake for one or more household members. Overall, 20 (42%) households were categorized as food secure, 18 (37%) as mildly Figure 1 Proportion of children who did not meet energy recommendations by age and CD4% or moderately food secure and 10 (21%) as severely food insecure. Milk and fish consumption were more frequent in food secure households. All 48 caregivers (100%) indicated that subjects consumed grains on a daily basis, including rice boiled with oil (73%), maize ugali(60%), and mixed flour porridge (41%). More saturated fat (e.g., from cooking oil) was consumed on average by subjects whose households were classified as food secure than subjects from households with some level of food insecurity.
Discussion
In 60% of the children in our study, energy intake was below recommended values. A wide spectrum of micronutrient deficiencies was documented and more than 50% of households reported some degree of food insecurity. We found 38% of children to be stunted, 27% to be underweight and 6% to be wasted.
It is estimated that 44% of children under the age of five are moderately to severely stunted while 17% and 7% suffer from severe underweight and wasting, respectively in Tanzania.[2] Furthermore, a study among children in an age range similar to our cohort demonstrated that HIV-positive children were four times more likely to be underweight and almost 10 times more likely to be wasted as HIV-negative children.[23] In our study, anthropometric z-scores were lower for children who did not meet WHO energy recommendations compared to those who met the recommendations.
When considering intake from diet alone, over half of the children had inadequate intakes of 11 of the 13 micronutrients surveyed in this study including many that are essential for optimal growth and immune function. While children with better growth had high-er energy intakes, this appeared to come from foods higher in fat and sugar as protein intake was similar to malnourished children; they also had inadequate in-takes of many micronutrients. This underscores the need for improved dietary diversity in children living with HIV with an emphasis on a balanced diet with nutrient dense foods that are culturally appropriate.
The majority of children in our study (88-100%) did not meet the recommended dietary intake of vitamin E, calcium and vitamin D. Multiple other studies have documented deficiencies of these micronutrients in adults and children with HIV infection. [24,25] These micronutrients are important for effective immune function and deficiencies have been associated with increased risk of HIV disease progression including an increased risk of opportunistic infection.[26] Though we did not assess serum levels of micronutrients, they would be expected to be low and potentially affect absorption, pharmacokinetics, and toxicity of ART.[27] Children who were stunted or underweight had lower dietary intake of vitamin D and vitamin E. Bueno et al also reported low dietary intake of vitamin D in Brazil among stunted children and adolescents regardless of their HIV status.[28] A study done in pregnant women in Tanzania showed that when infants of mothers with low vitamin D levels, they were 29% more likely to be stunted and 33% more likely to be underweight than infants of mothers with normal vitamin D levels. [29] These micronutrients play a critical role in optimal growth and development of the child.
Collectively, our findings suggest that the choice of routine micronutrient in our clinic should be revalu-ated. The multivitamin currently being used indicates that routine supplementation can be effective in re-ducing dietary micronutrient deficiencies, as was seen for thiamine and riboflavin. However, our standard multivitamin targets micronutrients that had low di-etary deficiency rates within our population (vitamins C and B6), and omits the majority of micronutrients that have high rates of dietary deficiency.
We found that more than half of the study house-holds were food insecure. Other studies have also reported high levels of food insecurity among HIV in-fected individuals living in low-income families in de-veloping countries, especially in families with young children.[30] Food insecurity is likely to be an important contributing factor to the dietary deficiencies observed. Although we did not look at the association between food insecurity and ART compliance, other studies have reported a significant association between food insecurity and ART non-adherence.[31]
Study Limitations. There are several limitations of the present study including the small convenience-based sample. However, we did select children from a range of ages and HIV disease severity. We did not have a means to accurately assess if children were taking the multivitamin supplements they were provided nor did we measure serum levels of micronutrients. Thus, though our calculations of dietary micronutrient intake are likely to be valid, our estimates of combined dietary and supplemental micronutrient deficiency may underestimate defici-encies for the 4 micronutrients in the supplement that were quantified in this study.
Conclusions and Public Health Implications
We found growth deficiencies as well as significant dietary energy and micronutrient deficits among HIV-infected children under care in a specialty HIV program which provided routine nutritional coun-seling and a vitamin supplement. These findings sug-gest a need for enhanced dietary education with an emphasis on a culturally appropriate, diverse diet of high nutrient density, provision of macronutrient supplementation to children with energy deficient diets, and routine administration of a broader mi-cronutrient supplement to HIV-infected children in resource limited regions.
Financial Disclosure:
None;
Acknowledgements:
The authors acknowledge the following for their assistance with this study: Janeth Naubutu and other DPP clinic nurses and staff, and the many Tanzanian children and their caregivers who volunteered for this study.
Conflicts of Interest:
No authors have financial interests that pose a conflict of interest.
Funding Support:
This work was supported by the Fogarty International Center, Foundation for Treatment of Children with AIDS [D43-TW006807].
References
- Child mortality in relation to HIV infection, nutritional status, and socio-econom-ic background. International Journal of Epidemi-ology. 2005;34:61-68.
- [CrossRef] [PubMed] [Google Scholar]
- Statistics for Tanzania, United Repub-lic of. 2009. Available at: http://www.unicef.org/infobycountry/tanzania_statistics.html (accessed )
- [Google Scholar]
- Maternal perceptions of factors contributing to severe under-nutrition among children in a rural African setting. Rural Remote Health. 2011;11:1423.
- [CrossRef] [PubMed] [Google Scholar]
- Constraints on good child-care practices and nutritional status in urban Dar-es-Salaam, Tanzania. Food Nutrition Bulletin. 2006;27:236-244.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of structural adjustment policies on women's and children's health in Tanzania. Review of African Political Economy. 1995;22:43-53.
- [CrossRef] [PubMed] [Google Scholar]
- Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2000;31:803-805.
- [CrossRef] [PubMed] [Google Scholar]
- Energy ex-penditure and wasting in human immuno-deficiency virus infection. The New England Journal of Medicine. 1995;333:83-88.
- [CrossRef] [PubMed] [Google Scholar]
- HIV-related diarrhea is multifac-torial and fat malabsorption is commonly pres-ent, independent of HAART. Ameri can Journal of Gastroenterology. 2001;96:1831-1837.
- [CrossRef] [PubMed] [Google Scholar]
- Mi-cronutrients in HIV-positive persons receiving highly active antiretroviral therapy. The Ameri-can Journal of Clinical Nutrition. 2007;85:333-345.
- [CrossRef] [PubMed] [Google Scholar]
- HIV and mal-nutrition: effects on immune system. Clinical and Developmental Immunology 2012:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pneumonia and malnutrition are highly predictive of mor-tality among African children hospitalized with human immunodeficiency virus infection or ex-posure in the era of antiretroviral therapy. The Journal of Pediatrics. 2011;159:484-489.
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional rehabilitation of HIV-exposed infants in Malawi: results from the drug resources enhancement against AIDS and malnutrition program. Inter-national Journal of Environmental Research and Public Health. 2012;9:421-434.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin A and vita-min B-12 concentrations in relation to mortality and morbidity among children born to HIV-in-fected women. Journal of Tropical Pediatrics. 2010;56:27-35.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of intestinal parasitic infections on the nutritional status of rural and urban school-aged children in Nigeria. International Journal of MCH and AIDS. 2012;1:73-82.
- [CrossRef] [PubMed] [Google Scholar]
- WHO child growth standards and the identification of severe acute malnu-trition in infants and children. In: Report of a Joint WHO/United Nation's Children's Fund. Gene-va: WHO; 2009.
- [Google Scholar]
- An overview of USDA's Dietary Intake Data System. Journal of Food Composition and Analysis. 2004;17:545-555.
- [CrossRef] [Google Scholar]
- Tanzania food composition tables. Dar es Salaam: Muhimbili University of Health and Allied Sciences (MUHAS)/Har- vard School of Public Health (HSPH); 2008.
- [Google Scholar]
- Human energy require-ments. In: Report of a Joint FAO/WHO/UNU Ex-pert Consultation. Rome: Food and Agricultural Organization; 2004.
- [Google Scholar]
- Protein and amino acid re-quirements in human nutrition. In: WHO Techni-cal Report Series 935. Geneva: WHO; 2007.
- [Google Scholar]
- Human Vitamin and Mineral Re-quirements. In: Report of a joint FAO/WHO ex-pert consultation. Rome: FAO/WHO; 2002.
- [Google Scholar]
- Guidelines for an Integrated Approach to the Nutritional care of HIV-infected children (6 months-14 years) Geneva: WHO; 2009.
- [Google Scholar]
- Food and Nu-trition Technical Assistance Project (FANTA): Household Food Insecurity Access Scale (HFI- AS) for Measurement of Food Access: Indicator Guide (v.3) Washington, D.C: Food and Nutrition Technical Assistance Project; 2007.
- [CrossRef] [Google Scholar]
- Undernutrition among HIV-positive children in Dar es Salaam, Tanzania: antiretroviral therapy alone is not enough. BMC Public Health. 2011;11:869.
- [CrossRef] [PubMed] [Google Scholar]
- US youths in the early stages of HIV disease have low intakes of some micronutrients important for optimal immune function. Journal of the American Dietetic Asso-ciation. 2004;104:1095-1101.
- [CrossRef] [PubMed] [Google Scholar]
- HIV in-fection is associated with decreased dietary di-versity in South African children. The Journal of nutrition. 2008;138:1705-1711.
- [CrossRef] [PubMed] [Google Scholar]
- Pos-sible benefits of micronutrient supplementation in the treatment and management of HIV infection and AIDS. African Journal Pharmacy Phar-macology. 2009;3:404-412.
- [Google Scholar]
- Nutritional considerations in the use of ARV/HAART in resource-limited settings. In: Consultation on Nu-trition and HIV/AIDS in Africa: Evidence, les-sons, and recommendations for action. Geneva: WHO; 2005.
- [Google Scholar]
- The 24-hour recall for the assessment of dietary calcium, phospho-rus and vitamin D intakes in stunted children and adolescents. Revista de Nutricao: Brazilian Journal of Nutrition. 2010;23:65-73.
- [Google Scholar]
- Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed chil-dren in Tanzania. The Pediatric Infectious Dis-ease Journal. 2012;31:171-175.
- [CrossRef] [PubMed] [Google Scholar]
- Food insecurity and hunger are prevalent among HIV-positive indi-viduals in British Columbia, Canada. The Journal of Nutrition. 2005;135:820-825.
- [CrossRef] [PubMed] [Google Scholar]
- Food insufficiency and medication adherence among people living with HIV/AIDS in urban and peri-urban settings. Prevention Science. 2011;12:324-332.
- [CrossRef] [PubMed] [Google Scholar]





