Translate this page into:
Pregnancy and Birth Outcomes Among Women on Antiretroviral Therapy: A Long-term Retrospective Analysis of Data from a Major Tertiary Hospital in North Central Nigeria
*Corresponding author email: daparm@unijos.edu.ng
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background and Objective:
Antiretroviral therapy (ART) has transformed human immune deficiency virus (HIV) infection from a death sentence to a chronic syndrome, allowing infected individuals to lead near-normal lives, including achieving pregnancy and bearing children. Notwithstanding, concerns remain about the effects of ART in pregnancy. Previous studies suggested contradictory associations between ART and pregnancy. This study determined birth outcomes in pregnant women who accessed ART between 2004 and 2017 at a major tertiary hospital in North Central Nigeria.
Methods:
This was a retrospective study of 5,080 participants. Ethical clearance was obtained from the Institutional Review Board of the Harvard T. H. Chan School of Public Health Boston. A pro forma for data abstraction was designed and used to collect data. Abstracted data were sorted and managed using SPSS® version 22. The Chi-square test was used to calculate the proportions of pregnancy outcomes. One-way analysis of variance was used to test the effect of antiretroviral drug regimens on mean birth weight and gestational age at delivery. All levels of significance were set at p 0.05.
Results:
Pregnancy outcomes were recorded as live birth (99.8%), stillbirth (0.2%), preterm delivery (6.6%), and low birth weight (23%). There was a statistically significant association between ART in pregnancy and low birth weight {χ2 [(5, n = 3439) = 11.99, p = 0.04]}. The highest mean birth weights were recorded in participants who received drug combinations with protease inhibitors or efavirenz, in contrast to participants who received Nevirapine, stavudine and Emtricitabine/Tenofovirbased regimens. However, there was no significant difference in the gestational age of babies at birth for the six ART regimens in the study.
Conclusion and Global Health Implications:
Findings support the benefits of ART in pregnancy, which is in line with the testing and treatment policies of the 90-90-90 targets for ending HIV by the year 2030.
Keywords
Pregnancy
Outcomes
Stillbirth
Preterm
Low-Birth Weight
Women
Antiretroviral
Therapy
HIV
PMTCT
Jos
Nigeria
1. Introduction
1.1. Background of the Study
With the advent of pharmacotherapy, the scenario in which infection with the Human Immune Deficiency virus (HIV) was almost a certain death sentence has significantly improved, with the advent of pharmacotherapy. Infection with the disease has undergone significant transitions in terms of morbidity and mortality, to the extent that individuals infected with HIV can now lead a near-normal life. Currently, over 40 therapeutic agents have been approved and are in use, either as single tablet or fixed dose regimens of highly active antiretroviral therapy (HAART).1 Latest guideline recommends that ART should be initiated in all adults living with HIV, regardless of the clinical-stage (as described by the World Health Organization; WHO) and at any CD4 cell count.2 By 2017, the Joint United Nations Program on HIV and AIDS (UNAIDS) reported almost 40 million individuals were living with HIV, of whom nearly 22 million were assessing antiretroviral therapy (ART).3 Hence, the natural history of the disease became altered with the development and subsequent utilization of HAART. This has led to a significant decline in the number of AIDS-related deaths. Globally, fewer than 1 million people annually die from AIDS-related illnesses as a result of sustained access to ART.4 Moreover, the WHO reported a 35% decline in new HIV infections between the year 2000 and 2015, a 45% reduction in the number of people dying from the disease in 2015 compared to 2005.5 This success was mainly driven by a global policy of expanding access to antiretroviral drugs (ARVS) for about 76% of all HIV positive pregnant women and to prevent mother-to-child transmission (PMTCT) of the virus.6 Guidelines for ART in pregnancy recommend initiation of drugs from 14 weeks or as soon as possible after that; this treatment should be continued throughout the patient’s life.2
PMTCT strategy in Jos University Teaching Hospital (JUTH) consists of a triple combination of ARVs in line with the Option B plus guidelines nationally adopted in Nigeria.6 Revised option B-plus guidelines recommended universal access to triple-drug regimens irrespective of CD4 cell count and WHO clinical disease stage.7 Benefits of the strategy include improved maternal outcomes and a transmission reduction of mother-to-child HIV to less than 2%; this improvement was against a background transmission risk of 35% in the absence of any interventions and with continued breastfeeding.6,8 This was demonstrated in a study that followed the HIV transmission status of infants delivered to HIV-positive mothers in JUTH up to 18 months post-delivery.9 The authors in a retrospective observational study of 996 HIV-exposed neonates found a lower risk of transmission in mothers that were on ART prior to pregnancy as compared with those who only started ART after pregnancy.
Despite the benefits of ART, some concerns exist, especially regarding pregnancy outcomes associated with the ARV regimens. Although the literature suggests the relative safety of ART in pregnancy, majority of the evidence is at best inconclusive, with a clear need for further studies.10 This is more so for regimens that contain protease inhibitors (PIs), where the evidence for superior efficacy over others is not so strong.10,11 Similarly, revised guidelines by the WHO in 2016 recommended the use of an integrase inhibitor (INSTI), dolutegravir (DTG), as a preferred first-line for people living with HIV.12 This revision could potentially have an impact on the safety profile of ARVs in pregnancy.
1.2. Objectives of the Study
The objective of this study was to determine pregnancy outcomes associated with ART use in women who accessed ART from Jos University Teaching Hospital between 2004 and 2017.
1.3. Specific Aims and Hypothesis
To determine the proportion of stillbirth, gestation age at birth and birth weight and to determine if there is an existing association between ART during pregnancy and the identified outcomes. We hypothesized that there would be no difference in pregnancy outcomes across the various ART regimens used in the study cohort.
We hypothesized that there would be no difference in pregnancy outcomes across the various ART regimens used in the study cohort.
2. Methods
2.1. Study Variables
The outcome variables were as follow: low birth weight (LBW) was defined as birth weight less than 2500g; pre-term delivery (PTD) was defined for babies born before 37-week gestation calculated from the first day of the last menstrual period, and stillbirth (SB) was measured by fetal death after 20-week gestation. The independent variables were ART regimens consisting of various triple combinations of ARVs.
The study was conducted in the AIDS Prevention Initiative in Nigeria (APIN) Program. The program administers the President’s Emergency Plan for AIDS Relief (PEPFAR) fund through a cooperative agreement with Harvard T.H. Chan School of Public Health Boston, the United States Department of Health and Human Services, and the Center for Disease Control and Prevention (CDC).
Data Collection
Data were extracted by the APIN data manager from the electronic records of patients; the record was held in the Harvard PEPFAR database and Repository Bank and transferred to a pro forma, which was designed on Microsoft® Excel 2010 (Microsoft Inc. USA) for the project (Appendix 1). Data were extracted, cleaned, and sorted by the first author MPD onto a database in IBM’s statistical package for the social sciences SPSS® version 22 (IBM, SPSS Inc USA). Entries were checked for accuracy and completeness by BNJ and RCO and supervised by MEB, MPC, and JM.
2.2. Statistical Analysis
Descriptive statistics comprising of frequencies and percentages were calculated for birth outcomes. The Chi squared test for independence was used to test for the association between SB, PTD, and LBW with each of the ART regimens. One-way analysis of variance was used to test ART’s effect on mean birth weight and mean gestational age at delivery. All levels of significance were set at p < 0.05.
2.3. Ethical Approval
Approval was obtained from the Institutional Review Board of the Harvard T. H. Chan School of Public Health Boston. All participants in the Harvard/APIN PEPFAR program provided informed consent for use of their data. Additionally, administrative permission was sought from the management of APIN and JUTH to extract patient records. No identifying information was collected from the records, and all anonymized data collected from patient records were treated with utmost confidentiality; only aggregated data was reported.
3. Results
3.1. Sociodemographic Characteristics
Records from a total of 5,080 HIV-positive pregnant women were abstracted and analyzed. A breakdown of maternal demographic characteristics showed that the participants’ mean age in the study was 32 ± 5 years. The majority (70%) of the participants comprised nearly equal proportions of the unemployed and traders/skilled artisans. Similarly, 70% of them had completed up to secondary school level of education. The greatest proportion of the study population (slightly over 90%) had used ART before pregnancy, and more than 57% of them were initially treated with zidovudine (AZT), Lamivudine (3TC), and Nevirapine (NVP). Detailed characteristics of study participants are presented in Table 1.
| Characteristic | Frequency | Percentage |
|---|---|---|
| Age group n = 4916 | ||
| 15-19 | 31 | 0.6 |
| 20-24 | 392 | 8.0 |
| 25-29 | 1271 | 25.9 |
| 30-34 | 1765 | 35.9 |
| 35-39 | 1179 | 24.0 |
| 40 and Above | 278 | 5.7 |
| Occupation n =4486 | ||
| Unemployed | 1570 | 35.0 |
| Trade and skilled artisans | 1548 | 34.5 |
| Civil service and administrative | 812 | 18.1 |
| Student | 550 | 12.3 |
| Others | 6 | 0.1 |
| Highest Educational level attained n= 4505 | ||
| No formal education | 437 | 9.7 |
| Primary level | 892 | 19.8 |
| Secondary level | 1834 | 40.7 |
| Tertiary level | 1342 | 29.8 |
| ART use before Pregnancy n = 5080 | ||
| Yes | 4552 | 89.6 |
| No | 528 | 10.4 |
| ART Regimen initiated n =4400 | ||
| AZT, 3TC and NVP | 2513 | 57.1 |
| EFV Based | 440 | 10.0 |
| dT4, 3TC and NVP | 498 | 11.3 |
| TRUVADA Based | 814 | 18.5 |
| ABC, 3TC and NVP | 84 | 1.9 |
| PI Based | 51 | 1.2 |
Notes: ART= Antiretroviral Therapy; AZT=Zidovudine; 3TC= Lamivudine;
NVP= Nevirapine; PI-Protease Inhibitor.
3.2. Birth Outcomes
Birth outcomes were recorded for 4,117 participants, out of which 4,106 (99.8%) were live births, and 11 (0.2%) were stillbirths. About 4,000 participants had their mode of infant delivery recorded of whom the majority 2,879 (72.0%) were by spontaneous vaginal delivery. There were 12 assisted vaginal deliveries and one home birth. Of the over 4,000 recorded live births, the majority were at term and had normal birth weights (Table 2). Moreover, 435 infants from the study participants were screened for HIV status, of which 417 (95.9%) were negative, and 18 (4.1%) had no recorded results. Other infants’ birth characteristics are shown in Table 3.
| Birth Characteristic | Frequency | Percent |
|---|---|---|
| Type of delivery n = 3693 | ||
| Term delivery | 3448 | 93.4 |
| Pre-term delivery | 245 | 6.6 |
| Birth weight n =3918 | ||
| Low birth weight (<2500g) | 916 | 23.4 |
| Normal birth weight (2500-4000g) | 3002 | 76.6 |
| Characteristic | N | Minimum | Maximum | Mean | Sth. Deviation |
|---|---|---|---|---|---|
| Infant Length (cm) | 3493 | 10.00 | 58.50 | 47.9309 | 3.65822 |
| Head Circumference (cm) | 2197 | 10.00 | 46.60 | 33.9593 | 3.76309 |
| Gestational age at delivery (weeks) | 3771 | 30.0 | 45.0 | 38.357 | 1.4263 |
3.3. Association between ART during pregnancy and birth outcomes
There was no statistically significant association between pre-term delivery (PTD) and ART regimen: χ2 [(5, n= 3,222) = 7.34, p=0.12]. In contrast, the chi-square test for independence revealed a statistically significant association between low birth weight and the ART regimen used by the mother. χ2 [(5, n= 3,439) = 11.99, p= 0.04]. Similarly, chi-square analysis for categorical variables also revealed a statistically significant association between ART regimen and gestational age at delivery. These relationships were further explored in parametric analysis to determine the effects of specific ART regimens on these birth outcomes.
Effect of ART regimens on birth weight
Mean birth weight for babies in the study (n = 3,451) was 2.95 kg ± 0.56 kg. There was a statistically significant difference p < 0.05 in the mean birth weight for the six ART regimens: F (5, 3450) = 4.524, p = 0.000). Despite reaching statistical significance, the actual difference in mean birth weight for the ART regimens was very small. The effect size was calculated using eta squared (sum of squares between groups/total sum of squares; 6.987/1071.00 = 0.01).
Post-hoc analysis using Turkey HSD test indicated that the mean birth weight for efavirenz (EFV) based regimens (mean = 3.07 ± 0.62 kg) was significantly greater than the mean birth weight for AZT, 3TC, and NVP regimen (mean = 2.94 ± 0.62 kg). Similarly, mean birth weight for EFV based regimen (mean = 3.07 ± 0.62 kg) was significantly greater than the mean birth weight for Stavudine (dT4), 3TC, NVP regimen (mean = 2.93 ± 0.55 kg), and Truvada based regimen (mean = 2.93 ± 0.60 kg). In contrast, Abacavir (ABC) based regimen had a mean birth weight of 2.97 ± 0.58 kg, and protease inhibitors (PI) based regimen had a mean birth weight of 3.13 ± 0.66kg, which did not significantly differ from the mean birth weight of babies born to mothers on EFV based therapies Figure 1.
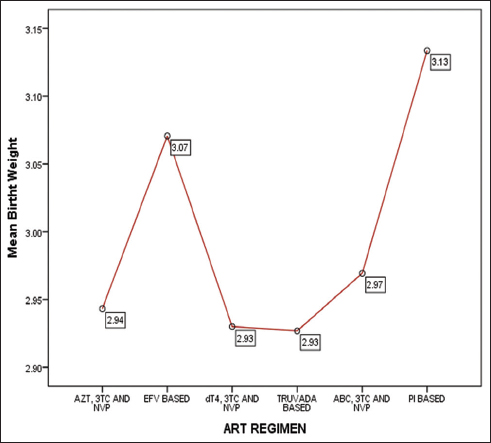
- Mean Birth weights of babies according to the ART regimen of mothers
- Notes: AZT=Zidovudine; 3TC= Lamivudine; EPV=; d4T=Stavudine; ABC=Abacavir; PI=Protease Inhibitors.
- Change spelling dT4 to d4T.
4. Discussion
4.1. Proportion of Pregnancy Outcomes in the Study Cohort
The low proportion of SB in this study supports the growing evidence of ART’s positive effect on birth outcomes.12–14 Indeed, HIV infection on its own in pregnant women is associated with a doubled risk of SB.15 Hence, the researchers’ finding may be a reflection of ART’s beneficial effect. This result was in consonance with findings of several other studies in sub-Saharan Africa that reported that a proportion of SB to be in a range of 2.5% - 10%.12,13 Moreover, ART’s positive benefits in reducing SB has been demonstrated in clinical trials, such as the study by Stringer et al.14 in which data from 253 female participants were analyzed; the women became pregnant while participating in three clinical trials: the first study was used by researchers to compare a protease inhibitor-based regimen against a non-nucleoside reverse transcriptase inhibitor-based regimen; in the second study, researchers tested a once-a-day PI-based regimen versus an EFV-based regimen for initial treatment of HIV infection, and in the third study, researchers tested immediate versus delayed ART for prevention of sexual transmission of HIV-1 among serodiscordant couples. Stringer et al. recorded 11 episodes of SB across the three trials, equivalent to 4% of the overall study population. 14 Similarly, Moodley et al. reported a reduction in the odds of SB for mothers receiving ART compared to mothers who did not receive any ART.16
Preterm delivery in this study was 6.6%, which was lower than 10.4% as reported by Chetty et al.17 Other studies documented higher proportions of preterm delivery, up to 22% in HIV-infected mothers vs 13% in uninfected mothers.18 Similarly, our findings contrasted those of Stringer et al., who reported high rates of preterm delivery in their study.14 This discrepancy may have arisen because the study by Stringer et al. was a randomized controlled trial with strict inclusion/exclusion criteria that could have over-selected other predisposing factors for preterm delivery in contrast to our study (which was observational, less restrictive in the inclusion of participants, and conducted under a real-world clinical practice scenario). With regards to low birth weight (LBW), the prevalence was 23 % in our study compared with 21% reported by Zash et al.19
Association of Pregnancy Outcomes with ART Regimens
Our findings with regards to the association between SB and ART regimen and the association between PTD and ART regimen were not remarkable. The insignificant association between the two outcomes and ART does not necessarily imply a lack of ART’s effect. Rather, the explanation may be attributed to multiple factors, including the fact that the disease process on its own, rather than ART, has been shown in several studies to influence birth outcomes.15,16,20,21 These factors could have confounded our results. Notwithstanding, the benefits of ART, underscored by the relatively low proportions of adverse birth outcomes, may support the option B-plus strategy of initiating triple ART regimen as-soon-as-possible in pregnancy both for PMTCT and for the mother’s health.
Our findings extend to the inconclusive evidence found in the literature of the association between ART and adverse birth outcomes. A seven-year prospective observational study of 3,314 women in 10 HIV-treatment centers (Tanzania) demonstrated an increased risk of adverse birth outcomes associated with the use of highly active antiretroviral therapy during pregnancy.22 The authors reported a statistically significant association between PTD and ART use prior to conception. The study noted that the rate of PTD in mothers who initiated ART before pregnancy was 38% against an overall rate of 29% in the study population.22 In contrast, other studies either did not report any association between the use of ART and PTD,18 or reported beneficial effects of ART in reducing PTD.16,17,23 A five-year retrospective study of 2,549 HIV-infected women who attended antenatal clinics in the KwaZulu-Natal region of South Africa between 2010 and 2015 was identified; the study reported a decline in PTD from 20% in 2010 to 7.7% in 2015 among women who initiated ART pre-conception.17 Also, a cross-sectional analysis of data (9,847 births) from maternity registers of a regional hospital in Durban, South Africa, showed that women who received any ART regimen had significantly and highly reduced odds of PTD compared to those who did not receive any ART.16 Therefore, the need to implement strict pharmacovigilance is as apt today as it is prior to the implementation of the option B-plus strategy for universal access to ART in pregnancy.
Our findings with regards to the beneficial effect of EFV-based regimens on birth weight corroborated the results of other studies.21,22 Specifically, the PIs have not proven to have any superior safety profile than to EFV-based regimens. Comparative studies did not find any difference between the two types of ART regimen in terms of adverse birth outcome.24 Notwithstanding, several other studies associated ART use with increased rates of adverse pregnancy outcomes, thus, reiterating the need for pharmacovigilance in ART. 22,25,26 This also means that until definitive evidence emerges, it is still expedient to maintain caution in ART administration, especially during pregnancy.
4.2. Strengths and Limitations of the study
A major strength of this study was the researchers analyzed real clinical data, which ensured that results were immediately transferable and useful in practice. Moreover, the inclusion of data over the study period (almost 10 years) ensured that various elements of clinical practice and experience were captured in the study to the extent that study findings reflected almost all possible scenarios encountered in clinical practice.
The main limitation of this study as with all other retrospective studies was the lack of complete data, and in some cases, even the available data was of doubtful quality. The incomplete data placed a limitation on the data analysis and interpretation of the results. Additionally, the absence of a comparative control group limited the type of inferences that could be made from the outcomes.
Recommendations for further studies
Study findings suggest a design of more robust prospective maternity surveillance studies to systematically evaluate the effect of ART on pregnancy outcomes, which can minimize the impact of confounding factors and allow clearer inferences on the study objectives. Moreover, with the evidence of ART regimens’ relative safety, it will be appropriate to design randomized control trials to provide a direct head-to-head comparison of ART regimens that are available for patient care in JUTH.
5. Conclusion and Global Health Implications
The researchers of this study identified relatively low proportions of adverse pregnancy outcomes of SB, LBW, and PTD among the study cohort. Additionally, findings support the benefits of ART in pregnancy, as there was no strong evidence of a link between the ART regimens and the observed adverse pregnancy outcomes.
Findings in this study were contextually consistent with reported outcomes in the literature. This emphasized the benefits of early initiation of antiretroviral drug therapy in pregnancy. There remains a gap in the evidence that would either confirm or disconfirm the hypothesis of no link between ART regimens and adverse pregnancy outcomes.
Implications for Policy and Clinical Practice
Results of the current study did not identify any evidence that would warrant a change from the status quo in terms of the policy. Findings support early initiation of ART as stated in the 90-90-90 objectives of ending HIV by the year 2030.27 Thus, the policy of early commencement and continuation of life-long ART in pregnancy as outlined in the PMTCT option B-plus strategy may continue. Notwithstanding, administration of ART, especially the newer integrase inhibitors, should be performed with necessary caution as more safety data become available.
In terms of practice, the study provided evidence to justify current clinical practice with regards to the use of ART in pregnancy. However, aspects of documentation and data storage may require some attention. Indeed, some limitations to the study findings rest in the fact that critical data to support decision-making were either lacking or of doubtful quality.
Consequently, clinicians and other members of the patient-care team need to conceptualize and consolidate the data type that needs to be stored for patients receiving care. This should be properly coordinated by the data management department to ensure data quality and usability.
Acknowledgments
Authors acknowledge the support of STAMINA in providing part funding, the management of APIN led by Professor Onche Agbaji, for facilitating access to data, and Mrs. Bola H. Olatunde, the Data Manager at the APIN Centre, for retrieving patient records.
Compliance with Ethical Standards
Conflicts of Interest: All authors declare no conflict of interest.
Financial Disclosure: All authors declare no financial benefits directly or indirectly from anyone connected to this project.
Funding/Support: The first author received partial funding support for the project from a grant administered by Support for Training and Mentoring of Academics in Nigeria (STAMINA). Participants in this research were funded in part by the US Department of Health and Human Services, Health Research Resources and Services Administration (U51HA02522), and Center for Disease Control and Prevention (CDC) through a cooperative agreement with APIN (PS001058).
Ethics Approval: This was obtained from the Institutional Review Board of the Harvard T. H. Chan School of Public Health Boston.
Disclaimer: This research was a product of the authors’ work and does not represent the official position of the University of Jos or Jos University Teaching Hospital.
References
- The comparative effectiveness of antiretroviral therapies for HIV :evidence to inform precision public health. Comp Eff Res. 2017;6(2):85-87. doi:10.2217/cer-2016-0093
- [Google Scholar]
- Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection:Recommendations for a Public Health Approach. World Health Organization; 2016. doi:10.1016/j.jped.2014.04.007
- Sexual &Reproductive Health. UNFPA - United Nations Population Fund. Published 2017; https: //www.unfpa.org/sexual-reproductive-health
- UNAIDS DATA 2018. UNAIDS. Published 2018; http: www.unaids.org
- Progress Report 2016:Prevent HIV, Test and Treat All WHO Support for Country Impact. World Health Organization. Published 2016; http: //www.who.int/hiv/pub/drugresistance/ewi-hivdr-2016/en
- A New Guidance on Prevention of Mother-to-Child Transmission of HIV and Infant Feeding in the Context of HIV. 2010;1400
- Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. World Health Organization. Published 2014; https: //www.who.int/hiv/pub/guidelines/arv2013/en
- Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants:Recommendations for a Public Health Approach. 2010.
- Mother- to- child transmission outcomes of HIV exposed infants followed up in Jos North Central Nigeria. Curr HIV Res. 2015;13(3):193-200. doi:10.2174/1570162x1303150506182534
- [Google Scholar]
- Antiretroviral therapy for pregnant women living with HIV or hepatitis B:A systematic review and meta-analysis. BMJ Open. 2017;7(9):1-17. doi:10.1136/bmjopen-2017-019022
- [Google Scholar]
- Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV:Interim Guidelines. Supplement to the 2016 Consolidated Guidelines on the Use of Antiretrovir. World Health Organization. Published December 2018; https: //apps.who.int/iris/bitstream/handle/10665/277395/WHO-CDS-HIV-18.51-eng.pdf?ua=1
- Timing of combination antiretroviral therapy (cART) initiation is not associated with stillbirth among HIV-infected pregnant women in Malawi. Trop Med Int Heal 2019 doi:10.1111/tmi.13233
- [Google Scholar]
- Use of antiretroviral therapy during pregnancy and adverse birth outcomes among women living with hiv-1 in low- and middle-income countries:a systematic review. J Acquir Immune Defic Syndr. 2018;79(1):1-9. doi:10.1097/QAI.0000000000001770
- [Google Scholar]
- Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS One. 2018;13(7) doi:10.1371/journal.pone.0199555
- [Google Scholar]
- Effects of HIV infection on maternal and neonatal health in southern Mozambique:A prospective cohort study after a decade of antiretroviral drugs roll out. PLoS One. 2017;12(6):e0178134. doi:10.1371/journal.pone.0178134
- [Google Scholar]
- Improved pregnancy outcomes with increasing antiretroviral coverage in South Africa. BMC Preganancy Childbirth. 2016;16:35. doi:10.1186/s12884-016-0821-3
- [Google Scholar]
- Preterm delivery and small-for-gestation outcomes in HIV-infected pregnant women on antiretroviral therapy in rural South Africa:Results from a cohort study, 2010-2015. PLoS One. 2018;13(2):e0192805. doi:10.1371/journal.pone.0192805
- [Google Scholar]
- Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol. 2017;46(5):1678-1689. doi:10.1093/ije/dyx136
- [Google Scholar]
- Reassuring birth outcomes with tenofovir/emtricitabine/efavirenz used for prevention of mother to child transmission of HIV in Botswana. J Acquir Immune Defic Syndr. 2016;71(4):428-436. doi:10.1097/QAI.0000000000000847
- [Google Scholar]
- Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr. 2017;171(10):e172222. doi:10.1001/jamapediatrics.2017.2222
- [Google Scholar]
- Antiretroviral therapy in relation to birth outcomes among HIV-infected women:A cohort study. J Infect Dis. 2016;213(7):1057-1064. doi:10.1093/infdis/jiv389
- [Google Scholar]
- Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana:an observational study. Lancet Glob Heal. 2018;6(7):e804-e810. doi:10.1016/S2214-109X(18)30218-3
- [Google Scholar]
- The impact of highly active antiretroviral therapy on obstetric conditions:A review. Eur J Obstet Gynecol. 2017;210:126-131. doi:10.1016/j.ejogrb.2016.12.008
- [Google Scholar]
- Efficacy and safety of lopinavir/ritonavir- versus efavirenz- based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. 2015;29(2):183-191. doi:10.1097/OGX.0000000000000256
- [Google Scholar]
- Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in botswana. J Infect Dis. 2012;206(11):1695-1705. doi:10.1093/infdis/jis553
- [Google Scholar]
- Benefits and risks of antiretroviral therapy for perinatal hiv prevention. Obstet Gynecol Surv. 2016;72(18):143-145. doi:10.1056/NEJMoa1511691
- [Google Scholar]
- 90-90-90:An Ambitious Treatment Target to Help End the AIDS Epidemic. UNAIDS. Published October 2014; https: //www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf





