Translate this page into:
Trends and Risk Factors for Leishmaniasis among Reproductive Aged Women in the United States
*Corresponding author email: deepa.dongarwar@bcm.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background and Objective:
Leishmaniasis, a neglected tropical disease, is endemic in several regions globally, but commonly regarded as a disease of travelers in the United States (US). The literature on leishmaniasis among hospitalized women in the US is very limited. The aim of this study was to explore trends and risk factors for leishmaniasis among hospitalized women of reproductive age within the US.
Methods:
We analyzed hospital admissions data from the 2002-2017 Nationwide Inpatient Sample among women aged 15-49 years. We conducted descriptive statistics and bivariate analyses for factors associated with leishmaniasis. Utilizing logistic regression, we assessed the association between sociodemographic and hospital characteristics with leishmaniasis disease among hospitalized women of reproductive age in the US. Joinpoint regression was used to examine trends over time.
Results:
We analyzed 131,529,239 hospitalizations; among these, 207 cases of leishmaniasis hospitalizations were identified, equivalent to an overall prevalence of 1.57 cases per million during the study period. The prevalence of leishmaniasis was greatest among older women of reproductive age (35-49 years), Hispanics, those with Medicare, and inpatient stay in large teaching hospitals in the Northeast of the US. Hispanic women experienced a statistically significant increased odds of leishmaniasis diagnosis (OR, 1.80; 95% CI, 1.19-4.06), compared to Non-Hispanic (NH) White women. Medicaid and Private Insurance appeared to serve as a protective factor in both unadjusted and adjusted models. We did not observe a statistically significant change in leishmaniasis rates over the study period.
Conclusion and Global Health Implications:
Although the prevalence of leishmaniasis among women of reproductive age appears to be low in the US, some risk remains. Thus, appropriate educational, public health and policy initiatives are needed to increase clinical awareness and timely diagnosis/treatment of the disease.
Keywords
Leishmaniasis
United States
Women
Healthcare Disparities
Minority Health
Reproductive Aged
HCUP NIS
1. Introduction
Leishmaniasis, classified as a neglected tropical disease (NTD) by the World Health Organization (WHO), is estimated to affect between 700,000 and 1 million people worldwide per year.1 Caused by infection with a protozoan parasite of the Leishmania species and transmitted via the bite of an infected female phlebotomine sandfly; leishmaniasis is typically categorized into three main clinical subtypes: (1) visceral, (2) cutaneous and (3) mucocutaneous.1 Globally, leishmaniasis is commonly found in parts of the tropics and subtropics of Asia, the Middle East, southern Europe, Mexico, Central America and South America.2 The disease tends to be more widespread among those living in poverty-stricken areas where malnutrition, poor housing and inadequate sanitation are pervasive; leishmaniasis has also been found to be associated with population displacement, urbanization, environmental changes and compromised immunity.3,4
Generally regarded as a disease of travelers in the United States (US), epidemiological data on leishmaniasis in humans within US is limited.2 According to the Centers for Disease Control and Prevention (CDC), the occurrence of leishmaniasis in the US is rare, with most cases occurring among immigrants, military personnel and travelers returning from leishmaniasis endemic areas.2 In recent years, a few studies have reported leishmaniasis as endemic to the US due to autochthonous infections, with the majority of cases reported in the state of Texas.5,6 Factors such as immigration, socioeconomic and environmental conditions, ease of travel, and the proximity of Texas to the southern US border (which is shared with Mexico) are suspected to be associated with the cases observed.6,7 Data from these studies suggest that leishmaniasis may be acquired more frequently in the US than previously suspected and is likely under-reported due to a lack of awareness and unmandated reporting at the state and federal levels.5 Therefore, gaining a better understanding of the disease patterns and risk factors for leishmaniasis in the US may help increase awareness among public health officials and health care providers.
Cutaneous leishmaniasis, the most common form affecting humans, typically causes skin lesions on exposed areas of the body and may result in life-long scars, disability or stigma.1 The majority of US cases identified have been cutaneous2 and recent data suggest that approximately 68% of US endemic cases of cutaneous leishmaniasis occurred among women.5 Females of any age can acquire the disease; however, leishmaniasis infection during pregnancy (particularly visceral), has been shown to be debilitating and associated with congenital transmission and fetal death.8-11 There is also concern that pregnant women may be more susceptible to infection due to the changes in cellular immunity that occur during pregnancy.10 Given that epidemiological data is limited in this area, we sought to add to the literature on national rates of leishmaniasis in the US. Thus, the aim of this study was to examine trends and risk factors associated with a leishmaniasis diagnosis among women of reproductive age in the US.
2. Methods
For this cross-sectional, population-based study, we utilized data collected by the Agency for Healthcare Research and Quality’s (AHRQ) Nationwide Inpatient Sample (NIS), a component of the Healthcare Cost and Utilization Project (HCUP).12 NIS is a publicly available healthcare database containing information regarding inpatient hospitalizations throughout the US. It contains a 20% systematic random sample of discharges from all HCUP-participating US community hospitals, excluding long-term acute care and rehabilitation hospitals.12 As of 2017, the NIS has data from more than 7 million inpatient stays each year and approximates more than 35 million hospitalizations when weighted. Clinical and non-clinical data from each hospital stay are available in the NIS, including patient sociodemographics, discharge status, expected payment source, and hospital characteristics (including size, type and location).12 The NIS database, as well as documentation and additional information are publicly available on the HCUP website13 The Institutional Review Board of Baylor College of Medicine designated this study exempt, as publicly available, de-identified data was utilized in the research.
2.1. Study Variables and Sample
NIS data on hospitalizations from January 1, 2002 to December 31, 2017 were analyzed. For this study, we identified women of reproductive age, defined as 15-49 years, with a leishmaniasis diagnosis based on International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Disease, 10th Revision, Clinical Modification/Procedure Coding System (ICD 10-CM/PCS) diagnosis and procedure codes. We scanned the discharge records for an indication of leishmaniasis using ICD-9-CM codes beginning with ‘085’ and ICD-10-CM codes beginning with ‘B55.’ It is important to note that although rare in the US, cutaneous leishmaniasis is the most common form/clinical subtype of leishmaniasis among humans. However, due to this rarity and the nature of ICD-9-CM and ICD-10-CM codes, estimates from this study are representative of all leishmaniasis diagnoses and do not differentiate between the clinical subtypes.
Sociodemographic and other clinical characteristics for individual records were also extracted from the database. Women were categorized by age into the following groups: 15-24 years, 25-34 years, 35-49 years. Race was defined as Non-Hispanic White (NH-White), Non-Hispanic Black (NH-Black), Hispanic and NH-Others. Discharge status was categorized as routine, transfer, died, against medical advice (AMA) and other. Income status was categorized into quartiles in ascending order from lowest to highest: 0th-25th percentile, 26th-50th percentile, 51st-75th percentile and 76-100th percentile. Primary Payer was designated as Medicare, Medicaid, private insurance, and self-pay. Hospital regions included Northeast, Midwest, South, and West. Hospital location and type were categorized as rural, urban-teaching, urban non-teaching; while bed size was designated as small, medium, or large. Per HCUP publishing guidelines for privacy protections, we suppressed the report of cell sizes less than or equal to 10.12,14
2.2 Statistical Analysis
Data analyses were performed using We utilized R version 3·5·1 (University of Auckland, Auckland, New Zealand) and RStudio Version 1·1·5001 (Boston, MA). First, we identified all hospitalizations of reproductive aged women. Next, we calculated the proportion and prevalence (cases per million) of leishmaniasis diagnosis among these women categorized by sociodemographic, primary payer, and hospital characteristics; and subsequently conducted Pearson’s chi-squared tests to assess differences in proportion. We then calculated the trend rates of leishmaniasis per million hospitalizations using Joinpoint Regression Program 4.6.0.0,15 which is typically used to assess varying trends of a given outcome over time and ascertain statistically significant changes in apparent trends using Monte Carlo simulation technique.15 The results of the Joinpoint regression models were represented in the form of average annual percentage change (AAPC) in the outcome over the study period and its 95% confidence intervals. Lastly, we performed logistic regression modeling to generate unadjusted and adjusted odds ratios for the association between our selected sociodemographic and hospital characteristics (i.e. risk factors) and leishmaniasis (i.e. outcome) after excluding missing information from all the covariates (effective sample size 131,472,644). All tests of hypotheses were two-tailed with a type 1 error rate set at 5%.
3. Results
3.1. Prevalence of Leishmaniasis
Of the 131,529,239 women of reproductive age (15-49 years) hospitalized in the US from 2002 through 2017, 207 women were diagnosed with leishmaniasis, yielding a prevalence of 1.60 per million women. Table 1 displays the sociodemographic and hospital characteristics of the women in the study by the diagnosis of leishmaniasis. With increasing age, we observed an increase in the prevalence of leishmaniasis—women aged 35-49 years had the highest prevalence (1.77 cases per million), while those 15–24 years had the lowest prevalence (1.12 per million). NH-White women made up the greatest proportion of those with leishmaniasis comprising 47.8% of cases, while Hispanic women comprised 26.1% of leishmaniasis cases. However, Hispanic women had the highest prevalence of leishmaniasis (about 2.71 per million) compared to their NH-White (1.62 per million) and NH-Black (0.78 per million) counterparts. The majority of cases were routinely discharged (85%), 5.3% were transferred to another facility, and the remaining cases were either discharged Against Medical Advice (AMA) or died. Of note, the prevalence of leishmaniasis was approximately eight times greater among those who died, compared to those who were discharged routinely (11.79 versus 1.46 per million).
| Characteristic | Leishmaniasis | ||||
|---|---|---|---|---|---|
| No | Yes | Prevalence | |||
| n | % | n | % | (cases/million) | |
| Age | |||||
| 15-24 years | 32061479 | 24.4% | 36 | 17.4% | 1.12 |
| 25-34 years | 50228981 | 38.2% | 84 | 40.6% | 1.67 |
| 35-49 years | 49238572 | 37.4% | 87 | 42.0% | 1.77 |
| Race | |||||
| NH-White | 61281613 | 46.6% | 99 | 47.8% | 1.62 |
| NH-Black | 19307961 | 14.7% | 15 | 7.2% | 0.78 |
| Hispanic | 19935735 | 15.2% | 54 | 26.1% | 2.71 |
| Other | 9314062 | 7.1% | Suppressed* | ||
| Discharge Status | |||||
| Routine | 120703994 | 91.8% | 176 | 85.0% | 1.46 |
| Transfer | 4147369 | 3.2% | 11 | 5.3% | 2.65 |
| Died | 424181 | 0.3% | Suppressed* | ||
| Against Medical Advice | 1431112 | 1.1% | Suppressed* | ||
| Other | 4765781 | 3.6% | Suppressed* | ||
| Zip Income quartile | |||||
| 0th - 25th percentiles | 28415722 | 21.6% | 53 | 25.6% | 1.87 |
| 26th – 50th percentiles | 24053309 | 18.3% | 39 | 18.8% | 1.62 |
| 51st – 75th percentiles | 22443381 | 17.1% | 22 | 10.6% | 0.98 |
| 76th – 100th percentiles | 19406783 | 14.8% | 49 | 23.7% | 2.52 |
| Primary Payer | |||||
| Medicare | 9526408 | 7.2% | 34 | 16.4% | 3.57 |
| Medicaid | 40069940 | 30.5% | 43 | 20.8% | 1.07 |
| Private Insurance | 56526989 | 43.0% | 85 | 41.1% | 1.50 |
| Self-Pay | 11987318 | 9.1% | 24 | 11.6% | 2.00 |
| Hospital Region | |||||
| Northeast | 23431665 | 17.8% | 55 | 26.6% | 2.35 |
| Midwest | 28847363 | 21.9% | 33 | 15.9% | 1.14 |
| South | 51190049 | 38.9% | 72 | 34.8% | 1.41 |
| West | 28059956 | 21.3% | 46 | 22.2% | 1.64 |
| Hospital Bed Size | |||||
| Small | 16793862 | 12.8% | 21 | 10.1% | 1.25 |
| Medium | 35024564 | 26.6% | 54 | 26.1% | 1.54 |
| Large | 79252763 | 60.3% | 132 | 63.8% | 1.67 |
| Hospital Location and Teaching Status | |||||
| Rural | 14315384 | 10.9% | Suppressed* | ||
| Urban non-teaching | 48350379 | 36.8% | 62 | 30.0% | 1.28 |
| Urban teaching | 68405427 | 52.0% | 136 | 65.7% | 1.99 |
Leishmaniasis was least common among women in the 3rd quartile of zip code income (10.6%). Its prevalence was also lowest among those with Medicaid (1.07 per million) and patients with private insurance made up 41.1% of cases. While the greatest proportion of patients with leishmaniasis were treated in hospitals in the Southern US (34.8%), the Northeastern US had the highest prevalence of the disease (2.35 per million). Approximately two-thirds of all leishmaniasis cases were treated in large, urban teaching hospitals.
3.2. Trends in the Rates of Leishmaniasis
Figure 1 illustrates the trends in the rates of leishmaniasis per million hospitalizations of women of reproductive age over the 16-year study period. Peak incidences of the disease were seen in 2006, 2010, and 2016 with nadirs in 2005, 2009, and 2017. Joinpoint regression analysis demonstrated that the overall rate of leishmaniasis remained relatively stable over the study period, with no statistically significant change in average annual rates (AAPC: -0.6, 95% CI: -6.9, 6.1).
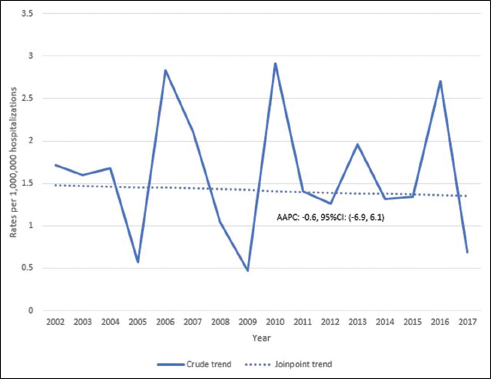
- Trends in the rates of leishmaniasis per million hospitalizations of women of reproductive age, United States, 2002-2017 Notes on figure legend: The X-axis represents the year of discharge and the Y-axis represents prevalence of leishmaniasis per million hospitalizations of women of reproductive age. Lines represent the trend estimated by joinpoint regression. Value represents the average annual percent change (AAPC), point estimate (95% confidence interval)
3.3. Risk Factors of Leishmaniasis
Table 2 presents the adjusted and unadjusted models of the odds ratio estimates for the association between leishmaniasis and various sociodemographic and hospital characteristics. Among women aged 35-49 years, the likelihood of having a leishmaniasis diagnosis was approximately 60% greater in the unadjusted model, compared to younger women (OR, 1.59; 95% CI, 1.06-3.84). However, after adjusting for the covariates (as indicated in the table), the difference observed by age, failed to reach statistical significance. With respect to race/ethnicity, Hispanic women experienced a statistically significant higher odds of being diagnosed with leishmaniasis (OR, 1.80; 95% CI, 1.19-4.06), than NH-White women.
| Characteristic | Leishmaniasis | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| OR* | p-value | OR | p-value | |
| Age | ||||
| 15-24 years | Reference | Reference | ||
| 25-34 years | 1.49 (0.61-3.62) | 0.38 | 1.33 (0.55-3.30) | 0.52 |
| 35-49 years | 1.59 (1.06-3.84)† | 0.03 | 1.28 (0.51-3.21) | 0.16 |
| Race | ||||
| NH-White | Reference | Reference | ||
| NH-Black | 0.49 (0.15-1.64) | 0.25 | 0.44 (0.13-1.52) | 0.19 |
| Hispanic | 1.69 (0.81-3.50) | 0.16 | 1.80 (1.19-4.06)† | 0.03 |
| Other | 0.67 (0.16-2.86) | 0.58 | 0.62 (0.14-2.65) | 0.52 |
| Discharge Status | ||||
| Routine | Reference | Reference | ||
| Transfer | 1.83 (0.44-7.64) | 0.41 | 1.70 (0.39-7.38) | 0.48 |
| Died | 5.38 (1.01-9.83)† | 0.04 | 6.24 (1.04-9.39)† | 0.04 |
| Against Medical Advice | 2.39 (0.33-5.46) | 0.39 | 2.24 (0.29-6.99) | 0.44 |
| Other | 1.38 (0.33-5.75) | 0.66 | 1.18 (0.27-5.08) | 0.83 |
| Zip Income quartile | ||||
| 0th - 25th percentiles | Reference | Reference | ||
| 26th – 50th percentiles | 0.88 (0.35-2.20) | 0.79 | 0.86 (0.34-2.18) | 0.75 |
| 51st– 75th percentiles | 0.52 (0.17-1.66) | 0.27 | 0.47 (0.14-1.56) | 0.22 |
| 76th – 100th percentiles | 1.36 (0.58-3.19) | 0.48 | 1.14 (0.46-2.83) | 0.77 |
| Primary Payer | ||||
| Medicare | reference | reference | ||
| Medicaid | 0.30 (0.11-0.81)† | 0.02 | 0.32 (0.11-0.92) † | 0.02 |
| Private Insurance | 0.42 (0.17-0.63)† | 0.04 | 0.46 (0.17-1.23) † | 0.04 |
| Self-Pay | 0.56 (0.18-1.75) | 0.35 | 0.6 (0.18-1.95) | 0.35 |
| Other | 0.42 (0.12-1.12) | 0.16 | 0.39 (0.11-1.35) | 0.14 |
| Hospital Region | ||||
| Northeast | Reference | Reference | ||
| Midwest | 0.49 (0.19-1.25) | 0.13 | 0.55 (0.18-1.62) | 0.28 |
| South | 0.60 (0.28-1.29) | 0.19 | 0.66 (0.3-1.45) | 0.31 |
| West | 0.69 (0.29-1.67) | 0.42 | 0.69 (0.28-1.69) | 0.42 |
| Hospital Bed Size | ||||
| Small | Reference | Reference | ||
| Medium | 1.20 (0.38-3.82) | 0.75 | 1.23 (0.38-3.98) | 0.73 |
| Large | 1.31 (0.45-3.78) | 0.62 | 1.4 (0.47-4.18) | 0.55 |
| Hospital Location and Teaching Status | ||||
| Rural | Reference | Reference | ||
| Urban non-teaching | 1.96 (0.44-8.73) | 0.38 | 1.85 (0.41-5.31) | 0.42 |
| Urban teaching | 3.04 (0.72-12.80) | 0.13 | 2.76 (0.65-6.75) | 0.17 |
Regarding discharge status, the adjusted odds of leishmaniasis among those who died during the inpatient stay was six times greater (OR, 6.24; 95% CI, 1.04-9.39), compared to those discharged routinely. One novel finding was that having Medicaid and Private Insurance appeared to serve as a protective factor in both unadjusted and adjusted models; women with either form of payment had an approximate 54-70% decreased likelihood of leishmaniasis. We observed no statistically significant differences in the associations of hospital characteristics (region, size, location and teaching status) and the diagnosis of leishmaniasis in women.
4. Discussion
Despite the peaks and troughs in leishmaniasis rates over the 16-year period covered in our study, our findings suggest that the overall prevalence of leishmaniasis among hospitalized women of reproductive age in the US has remained relatively low. Consistent with data reported by the CDC2 and prior research,5,6,16-18 our data suggest that the greatest proportion of leishmaniasis cases were in the Southern region of the US, but also geographically prevalent across the US. Additional research, including qualitative, observational, and case studies, are needed to provide an in-depth understanding of the factors contributing to the rate trends and geographic distribution of leishmaniasis in recent decades in the US. Nevertheless, at minimum, findings from this study are in support of the argument5 for mandatory reporting at the state and federal level.
The finding that Hispanic women were twice as likely to be diagnosed with leishmaniasis compared to White women is unclear. Considering that the clinical manifestations of leishmaniasis may not occur until weeks or months after exposure,2 one potential reason for this observation may be related to the large number immigrating into the US from countries where leishmaniasis is more common. Data suggest that from 1980 to 2017, the Central American immigration population in the US grew approximately tenfold,19 and Hispanic immigrants represented the second largest racial/ethnic group arriving in recent years.20 Aside from immigration or recent travels, it is also possible that many Hispanics currently live and/or work in areas of the US where there is an increased risk for exposure due to the presence of Leishmania sandflies. Even so, additional research is needed to better elucidate the reasons for this finding.
Our data also suggest that women with Medicaid were less likely to be diagnosed with leishmaniasis. It is probable that women with Medicaid have socioeconomic constraints which inhibit or limit their ability to travel to leishmaniasis endemic areas. Another possible explanation for this finding is that Medicaid recipients in this study may have utilized preventive health services and/or outpatient medical care, which allows for earlier diagnosis and treatment of leishmaniasis, if acquired, prior to hospitalization. In contrast, we also found that women of reproductive age diagnosed with leishmaniasis were more likely to die prior to discharge, compared to those routinely discharged. One reason for this observation may be due to the severity of disease at presentation or misdiagnosis of leishmaniasis, coupled with inappropriate treatment. For example, visceral leishmaniasis can be fatal if not treated.1 Additional research is needed to further validate and better understand the factors contributing to the observed findings.
Despite its strengths—including the large sample size, use of standardized nationally-representative dataset and analysis over a 16-year period—this study has some limitations. First, the generalizability of our results may be limited. Given that individuals admitted to the hospital tend to be sicker and have more comorbid medical conditions than the general populace, our findings may not be fully representative of all women of reproductive age in the US with leishmaniasis, but rather those with access to care. Second, as a limitation of the NIS dataset, we were not able to account for other potential confounders such as educational level, travel history, social history, military status or immigrant status. Third, while we recognize that cutaneous is the more common form/subtype among humans, and that the subtypes (cutaneous, mucocutaneous and visceral leishmaniasis) have vastly different clinical presentations and consequences, we were not able to distinguish between the clinical subtypes in our analyses. Thus, given the rarity of the disease in the US, limitations of the dataset and nature of ICD-9-CM and ICD-10-CM codes, estimates from this study are representative of all leishmaniasis diagnoses among hospitalized women during this study period. Further, considering the under-detection and under-reporting of human leishmaniasis in the US, the estimates presented in this study may not reflect the true prevalence of leishmaniasis among women of reproductive age over the 16-year period.
5. Conclusion and Global Health Implications
In summary, our study underscores the need for further research about neglected tropical diseases in the US. Due to potential underreporting, it is likely that the prevalence of endemic human leishmaniasis in the US is higher than reported in this and prior research. Appropriate educational, public health and policy initiatives aimed at increasing clinical awareness and timely diagnosis/treatment of the disease may serve as a first step in improved identification and tracking of human leishmaniasis in the US.
Acknowledgments:
None.
Compliance with Ethical Standards
Conflicts of Interest: The authors declare no competing interests.
Financial Disclosure: Nothing to declare.
Funding/Support: Research funding was provided by the US Department of Health and Human Services, Health Resources and Services Administration for the Maternal and Child Health Pipeline Training Program: TSU-BCM Maternal and Child Health Student Training for Academic Readiness and Success (MCH STARS) Undergraduate Fellowship Program, Grant No: T16MC29831.
Ethics Approval: Not applicable.
Disclaimer: None.
References
- 2020. Leishmaniasis. World Health Organization; https: //www.who.int/news-room/fact-sheets/detail/leishmaniasis
- Centers for Disease Control and Prevention. https: //www.cdc.gov/parasites/leishmaniasis/epi.html Page Last Reviewed February 18, 2020
- 2021. Leishmaniasis. World Health Organization; https: //www.who.int/health-topics/leishmaniasis#tab=tab_1
- The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95(3):239-243. doi:10.1016/s0035-9203(01)90223-8
- [Google Scholar]
- Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154(9):1032-1039. doi:10.1001/jamadermatol.2018.2133
- [Google Scholar]
- Cutaneous leishmaniasis in Texas:A northern spread of endemic areas. J Am Acad Dermatol. 2008;58(4):650-652. doi:10.1016/j.jaad.2007.11.008
- [Google Scholar]
- Human migration and leishmaniasis-nn the move. JAMA Dermatol. 2016;152(4):373-374. doi:10.1001/jamadermatol.2015.4765
- [Google Scholar]
- A comparison of liposomal amphotericin B with sodium stibogluconate for the treatment of visceral leishmaniasis in pregnancy in Sudan. J Antimicrob Chemother. 2006;58(4):811-815. doi:10.1093/jac/dkl342
- [Google Scholar]
- Visceral leishmaniasis during pregnancy:A rare case report from Greece. PLoS Negl Trop Dis. 2017;11(2):e0005134. doi:10.1371/journal.pntd.0005134
- [Google Scholar]
- Visceral leishmaniasis in pregnant women from Rio Grande do Norte, Brazil:A case report and literature review. Rev Soc Bras Med Trop. 2019;52:e20180233. doi:10.1590/0037-8682-0233-2018
- [Google Scholar]
- Visceral leishmaniasis in pregnancy:a case series and a systematic review of the literature. J Antimicrob Chemother. 2005;55(2):229-233. doi:10.1093/jac/dkh538
- [Google Scholar]
- Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; www.hcup-us.ahrq.gov/nisoverview.jsp Published 2017
- Healthcare Cost and Utilization Project (HCUP). Rockville MD: Agency for Healthcare Research and Quality; https: //www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp Published December 17, 2019 Accessed August 1, 2020
- Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; https: //www.hcup-us.ahrq.gov/db/publishing.jsp Published July 28, 2020
- NIH National Cancer Institue. http: //surveillance.cancer.gov/joinpoint/ Published April 21, 2020
- An atypical case of autochthonous cutaneous leishmaniasis associated with naturally infected phlebotomine sand flies in Texas, United States. Am J Trop Med Hyg. 2020;103(4):1496-1501. doi:10.4269/ajtmh.20-0107
- [Google Scholar]
- Visceral leishmaniasis:clinical observations in 4 US army soldiers deployed to Afghanistan or Iraq, 2002-2004. Arch Intern Med. 2007;167(17):1899-1901. doi:10.1001/archinte.167.17.1899
- [Google Scholar]
- Cutaneous leishmaniasis of the lower lip in a United States soldier. J Oral Maxillofac Surg. 2008;66(7):1513-1515. doi:10.1016/j.joms.2007.12.045
- [Google Scholar]
- Central american immigrants in the United States in 2017. Migration Policy Institute. https: //www.migrationpolicy.org/article/central-american-immigrants-united-states-2017 Published August 15, 2019
- [Google Scholar]
- Key findings about U.S. immigrants. Pew Research Center. https: //www.pewresearch.org/fact-tank/2019/06/17/key-findings-about-u-s-immigrants/ Published 2019
- [Google Scholar]





