Translate this page into:
HIV Virologic Suppression and Mental Well-being in Adolescents and Young Adults Living with HIV

*Corresponding author: Merrian J. Brooks, Children’s Hospital of Philadelphia Global Health Center, Philadelphia, United States. E-mail: brooksm2@chop.edu;
-
Received: ,
Accepted: ,
How to cite this article: Burmen B, Kurtzman G, Olashore A, BaghirovaBusang L, Poku O, Morakanyane PS, et al. HIV virologic suppression and mental well-being in adolescents and young adults living with HIV. Int J MCH AIDS. 2025;14:e007. doi: 10.25259/IJMA_45_2024
Abstract
Background and Objective:
Human Immunodeficiency Virus (HIV) care programs in resource-limited settings reserve counseling and referral for individuals with identified mental illness for those with HIV virological treatment failure (VTF). Adolescence is a period that may increase the likelihood of internalizing psychiatric disorders (IPDs). We assessed the relationship between HIV VTF and symptoms of IPDs among adolescents and young adults (AYA) living with HIV in Gaborone, Botswana.
Methods:
A cross-sectional study was conducted in Botswana from December 2018 to December 2019 among AYA living with HIV aged 12–24 years. Logistic regression analysis was used to examine relationships between age, sex, and HIV VTF (≥400 copies/mL) and clinically relevant IPD symptoms, namely, depression (Patient Health Questionnaire-9 score of ≥10) and anxiety (Generalized Anxiety Disorder-7 score of ≥10).
Results:
Of 553 participants, most were aged 16–19 years (53%) with an equal sex distribution; the minority had VTF using HIV viral load (VL) cutoff levels of ≥400 copies/mL (11%). Close to one-sixth (15%) had clinical depression symptoms; participants aged 16–19 years and 20–24 years were more likely to have clinically relevant depression symptoms when compared to participants who were aged 12–15 years (odds ratio [OR] 3.160, 95% confidence interval [CI] 1.094–9.123 and OR 4.748, 95% CI 1.624–13.877, p = 0.0117, for participants aged 15– 19 years and 20–24 years, respectively). Participants with clinically relevant anxiety symptoms (11%) or both clinically relevant anxiety and depression symptoms (8%) did not differ from those without these symptoms by age, gender, or VTF status. Similar results were observed using HIV VL cutoff levels of <1000 copies/mL.
Conclusion and Global Health Implications:
HIV VTF may be a poor proxy for mental well-being among AYAs receiving HIV. Universal screening should be considered for AYA receiving care for HIV.
Keywords
Acquired Immunodeficiency Syndrome
Anxiety
Depression
Human Immunodeficiency Virus
Mental Health
Viral Burden
Youth
INTRODUCTION
Background of the Study
Botswana has approximately a third of the country’s 2.3 million population who are aged 10–24 years, and a Human Immunodeficiency Virus (HIV) prevalence of 5–10% among adolescents and young adults (AYA) within this age group as of 2019.[1] In Botswana, internalizing psychiatric disorders (IPDs) have been shown to affect up to 37% of adolescents living with HIV,[2] with 33% and 44.5% of AYA living with HIV reporting anxiety and depression symptoms, respectively.[3] IPDs have been associated with poor HIV treatment outcomes, poor care retention, as well as the potential spread of HIV.[4] Adolescents living with HIV are also likely to engage in multiple risky health behaviors which negatively influence their well-being.[5]
Routine screening for symptoms of mental health disorders does not occur in healthcare settings in Botswana due to a shortage of mental health professionals.[6] Usually, counseling and referral for individuals with identified mental illness is targeted to those with poor adherence and virological failure.[7] However, few studies consider how well viral load (VL) suppression or adherence predicts the likelihood of internalizing mental illness. As HIV care moves toward a holistic model of treatment where integrated care is the new gold standard, and prevention of non-communicable diseases is the goal, understanding the relationship between HIV virologic status (either virologic treatment failure [VTF]or suppression) and mental well-being is vital.
Study Objective
The objective of this study was to assess the relationship between HIV virologic status and IPD symptoms among AYA living with HIV in Botswana to determine whether targeted counseling and referral of AYA with VTF suitably caters to all AYA who have IPD symptoms.
METHODS
Design, Setting, and Period
This cross-sectional study is a secondary analysis of data from a larger study that explored the frequency of IPDs among AYA[3] at Botswana-Baylor Children’s Clinical Center of Excellence; the largest HIV-treatment facility providing HIV care and treatment for AYA in Gaborone, Botswana from December 2018, when the annual routine screening for anxiety and depression symptoms for AYA living with HIV was introduced, to December 2019.
Study Population
Study participants were AYA aged between 12 and 24 years, who had been receiving HIV care at the study site for at least 1 year and had undergone mental health and HIV VL assessment at least once during the study period. Participants who did not meet any of the above criteria were ineligible for inclusion.
Study Procedures
Screening for IDPs was self-administered on tablet computers in either English or Setswana. A clinical psychologist and a social worker were available to refer patients with severe symptoms of IDPs for further management as described by Brooks et al.[8]
Measures
Generalized anxiety disorder 7 (GAD-7)
We used previously validated clinically relevant cutoff scores of the GAD-7 among adults in the US of 5, 10, and 15 to represent mild, moderate, and severe anxiety, respectively.[3] “Clinically relevant” anxiety symptoms were defined as a combination of moderate and severe anxiety symptoms that required active intervention.[8]
Patient health questionnaire 9 (PHQ-9)
Common cutoff points of the PHQ-9 used in this study were 0–4 (no depression), 5–9 (mild), 10–14 (moderate), 15–19 (moderately severe), and ≥20 (severe depression symptoms).[3] The proportion of participants with “clinically relevant” depression was defined as those with a PHQ-9 score of ≥10 that required active intervention.[8]
HIV VL
Persons aged ≤16 years had a VL assessment every 3 months as part of the standard of care. Other patients had VL assessments done 6 monthly or 12 monthly.[7] We used any VL assessment within 6 months of screening for mental health symptoms because many youths did not have a recent VL; 23%, 24%, 37%, and 50% of participants had a VL done within 1, 3, 6, and 12 months of mental health screening, respectively. At the time of the study, HIV viral suppression was defined either at HIV-1 RNA level <400 copies/mL or <1000 copies/mL based on reference assay Roche TaqMan.[7]
Age
Study outcomes were examined for the entire cohort and stratified by age groups ranging from 12 to 15, 16 to 19, and 20 to 24 years based on the minimum age of consenting for HIV services of 12 years and a combination of the United Nations International Children’s Emergency Fund’s categorization of adolescents into younger adolescents (10–15 years) and older adolescents (16–19 years) and the World Health Organization’s definition of young adults (20–24 years).[9]
Data Collection
Demographic, mental health symptoms and VL data were extracted from a de-identified database of the parent study.[3]
Statistical Analysis
We assessed the relationship between participants’ characteristics (i.e., age group, sex, and HIV virological status-suppression or VTF using HIV VL cutoff of <400 copies/mL of blood) and the presence of anxiety or depression symptoms using common clinical cutoff scores for anxiety and depression, and clinically relevant categories of anxiety and depression symptoms using Chi-square statistics or Fisher’s exact test. Shapiro–Wilk’s test was used to test for normality. Skewed data (i.e., GAD-7 scores and PHQ-9 scores) were summarized using medians and interquartile ranges; the Wilcoxon rank-sum test was used to compare the medians between participants who had and did not have VTF.
Logistic regression analyses were done to describe participant characteristics associated with reporting clinically relevant anxiety, depression, and “anxiety or depression” symptoms[10] with variables that had a p-value of <0.2 being included in the multivariate model and variables that attained p < 0.05 retained in the final model. All tests were two-sided with an alpha level of 0.05. A two-way analysis of variance was used to explore whether age and sex, and age and VL, synergistically interact to influence the likelihood of reporting anxiety or depression symptoms based on GAD-7 and PHQ-9 scores, respectively. Sensitivity analysis was done using an HIV VL cutoff of <1000 copies/mL of blood.[11] Analyses were done using statistical analysis system (Version 9.2).[12]
Sample Size
Post hoc computation of an optimal sample size to detect a difference in prevalence of mental health diagnoses between those with VTF (13.2%) and without VTF (5.9%) of 7.3%[4] illustrated that a sample of 86 and 177 youth would be required in each group (with and without VTF). Based on historical VTF rates in this population in Botswana (47% among men living with HIV aged 15–24 years in 2018),[13] an anticipated sample size of 425 and 479 adolescents with and without VTF in our population of 904 AYA would be sufficiently powered to detect similar differences between the two groups.
RESULTS
Participant Selection
Of the 1433 AYA who were seen during the study period, 533 (36%) were deemed eligible after excluding 64 % who did not have an HIV VL done within 6 months of mental health screening. Only 29% of 12-15-year-olds were eligible for inclusion in the final analysis versus 71% who were not (p = 0.0007). Ineligible participants did not differ from eligible participants by sex, HIV VTF status, and the presence of IPD symptoms of varying severity levels (data not shown).
HIV Virological Assessment
Most (338/533; 63%) eligible participants had a VL done on the same day as mental health screening, while the rest had a VL assessment done up to six months before (12%) or after (24%) of mental health screening (data not shown).
Participant Characteristics
Most participants were aged 16–19 years (53%) with an equal sex distribution. A minority had VTF (11%). Prevalence of VTF was 8%, 9%, and 16% among participants aged 12–15 years, 16–19 years, and 20–24 years, respectively, (p = 0.0403) (Data not shown).
Anxiety and Depression Symptoms
Most (69%) participants had no anxiety symptoms while 20%, 8%, and 3% had mild, moderate, and severe anxiety symptoms, respectively. Thus, 11% had clinically relevant anxiety symptoms; 11% of them had VTF.
Most (59%) participants had no depression symptoms while 26%, 11%, 3%, and 1% had mild, moderate, moderately severe, and severe depression symptoms. Thus, 15% had clinically relevant depression symptoms ; 15% of them had VTF.
Most (81%) participants had no clinically relevant symptoms of anxiety nor clinically relevant depression symptoms, 4% had clinically relevant anxiety symptoms only, 7% had clinically relevant depression symptoms only, while 8% had both clinically relevant anxiety and clinically relevant depression symptoms; 10% of them had VTF. (data not shown).
Predictors of Clinically Relevant Symptoms
Age, sex, and VL were not predictive of clinically relevant anxiety symptoms [Table 1] or of both clinically relevant anxiety and depression symptoms [Table 2].
| Clinically relevant anxiety/Total 61/533 (11%) n/N(%) | Univariate analysis OR (95% CI) | p-value | |
|---|---|---|---|
| Age group | |||
| 12–15 years | 5/80 (6) | Ref | 0.2956 |
| 16–19 years | 34/280 (12) | 2.073 (0.783–5.489) | |
| 20–24 years | 22/173 (13) | 2.185 (0.796–5.998) | |
| Sex | |||
| Female | 33/265 (13) | 1.219 (0.733–1.806) | 0.4677 |
| Male | 28/268 (11) | Ref | |
| Viral load | |||
| <400 copies/mL | 54/473 (11) | Ref | 0.9539 |
| ≥400 copies/mL | 7/60 (12) | 1.025 (0.444–2.368) |
| Clinically relevant anxiety and depression symptoms±/Total 41/533 (8%) n/N(%) | Univariate analysis OR (95% CI) | p-value | |
|---|---|---|---|
| Age group | |||
| 12–15 years | 3/80 (4) | Ref | 0.2547 |
| 16–19 years | 21/280 (8) | 2.081 (0.604–7.162) | |
| 20–24 years | 17/173 (10) | 2.797 (0.795–9.832) | |
| Sex | |||
| Female | 20/265 (8) | Ref | 0.9005 |
| Male | 21/268 (8) | 1.041 (0.551–1.970) | |
| Viral load | |||
| <400 copies/mL | 37/473 (8) | 1.188 (0.408–3.458) | 0.7520 |
| ≥400 copies/mL | 4/60 (7) | Ref |
Participants aged 16–19 years and 20–24 years were more likely to have clinically relevant depression symptoms when compared to participants who were aged 12–15 years (odds ratio [OR] 3.160, 95% confidence interval [CI] 1.094–9.123 and OR 4.748, 95% CI 1.624–13.877, p = 0.0117, for participants aged 15–19 years and 20–24 years, respectively) [Table 3].
| Clinically relevant depression/Total 79/533 (15%) n/N(%) | Univariate analysis OR (95% CI) | p-value | Multivariate analysis OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Age group | |||||
| 12–15 years | 4/80 (5) | Ref | 0.0103 | Ref | 0.0117 |
| 16–19 years | 40/280 (14) | 3.167 (1.097–9.137) | 3.160 (1.094–9.123) | ||
| 20–24 years | 35/173 (20) | 4.819 (1.650–14.072) | 4.748 (1.642–13.877) | ||
| Sex | |||||
| Female | 34/265 (13) | Ref | 0.1994 | ||
| Male | 45/268 (17) | 1.371 (0.847–2.220) | |||
| Viral load | |||||
| <400 copies/mL | 67/473 (14) | Ref | 0.2335 | ||
| ≥400 copies/mL | 12/60 (20) | 1.515 (0.765–3.000) |
Relationship between VTF Status and GAD-7 and PHQ-9 Scores
The GAD-7 and PHQ-9 scores for participants with and without VTF were 3.0 (interquartile range [IQR] 1.0–5.5) and 2.0 (IQR 1.0–6.0) (p = 0.1338), and 4.5 (IQR 2.0–8.0) and 3.0 (IQR 1.0–7.0), (p = 0.0752), respectively (Data not shown).
Interactions between Age, Sex, VL, and GAD-7 Scores
There was no interaction between the effects of age category and sex on GAD-7 scores F ([2, 1427) = 0.09, p = 0.981]. Simple main effects analysis showed that age category (p = 0.011) but not sex (0.874) had a statistically significant effect on GAD-7 scores [Figure 1].
![Plots to display the mean GAD-7 scores for different age categories based on their sex. A two-way ANOVA was performed to evaluate the effects of age category and sex on GAD-7 scores. The results indicated a significant main effect for age category, F (2, [1427) = 4.501, p = 0.011, partial η2 = 0.006; no significant main effect for sex, F (1, 1427) = 0.025, p = 0.874, partial η2 = 0.000; and no significant interaction between age category and sex, F ([2, 1427) = 0.09, p = 0.981, partial η2 = 0.000]. ANOVA: Analysis of variance, GAD-7: Generalized anxiety disorder 7-item, η2: Eta squared -effect size measure.](/content/159/2025/14/1/img/IJMA-14-e007-g001.png)
- Plots to display the mean GAD-7 scores for different age categories based on their sex. A two-way ANOVA was performed to evaluate the effects of age category and sex on GAD-7 scores. The results indicated a significant main effect for age category, F (2, [1427) = 4.501, p = 0.011, partial η2 = 0.006; no significant main effect for sex, F (1, 1427) = 0.025, p = 0.874, partial η2 = 0.000; and no significant interaction between age category and sex, F ([2, 1427) = 0.09, p = 0.981, partial η2 = 0.000]. ANOVA: Analysis of variance, GAD-7: Generalized anxiety disorder 7-item, η2: Eta squared -effect size measure.
Post hoc testing indicated that GAD-7 scores were significantly higher for participants aged 20–24 years than for participants aged 12–15 years (p = 0.010) and for participants aged 15–19 years when compared to participants aged 12–15 years (p = 0.038), but did not differ between those aged 15–19 years and 20–24 years (p = 0.644) [Figure 2].
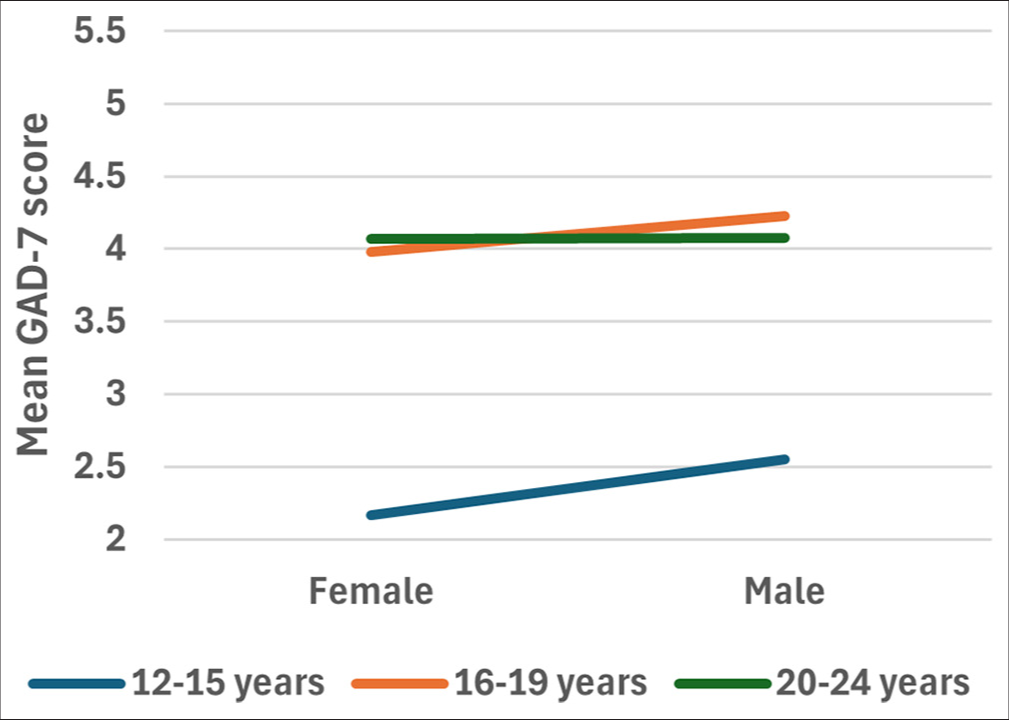
- Mean GAD-7 score for different age categories based on their sex. Post hoc testing using Tukey’s test indicated that GAD-7 scores were significantly higher for participants aged 20–24 years than they were for participants aged 12–15 years (p = 0.010). Likewise, post hoc testing using Tukey’s test indicated that GAD-7 scores were significantly higher for participants aged 15–19 years than they were for participants aged 12–15 years (p = 0.038). There was no significant difference between the GAD-7 scores of participants aged 15–19 years and participants aged 20–24 years (p = 0.644). GAD-7: Generalized anxiety disorder 7-item.
There was no interaction between the effects of age category and VL category on GAD-7 scores, F (2, 1427) = 0.151, p = 0.860). Simple main effects analysis showed that neither age group (p = 0.560) nor VL category (p =0.172) had a statistically significant effect on GAD-7 scores [Figure 3].
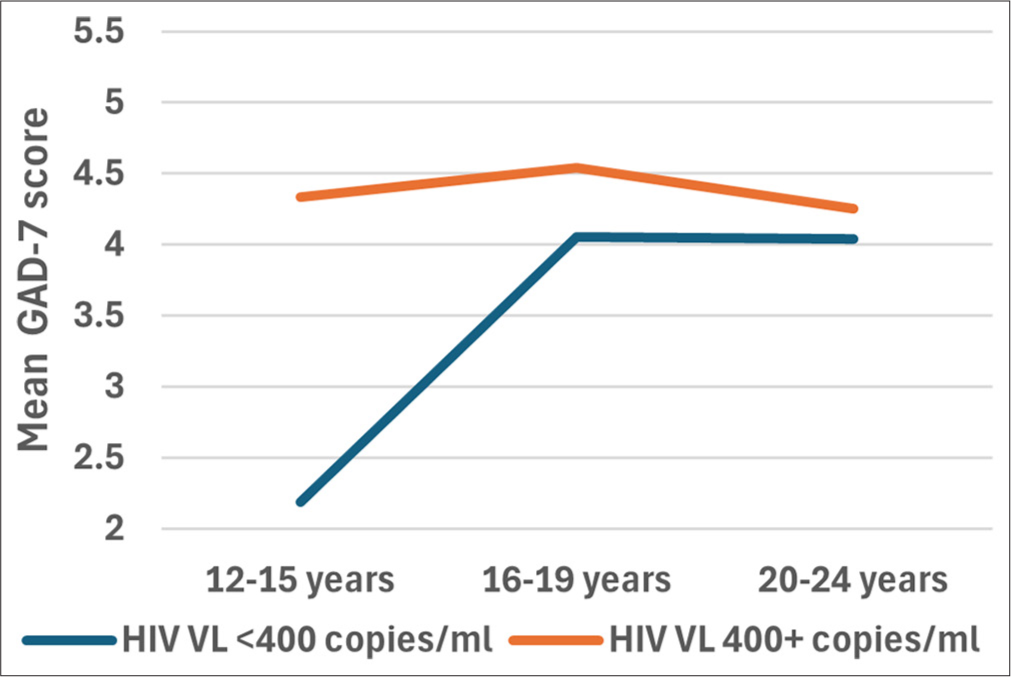
- Plots to display the mean GAD-7 scores for different age categories based on their viral load (VL). A two-way ANOVA was performed to evaluate the effects of age group and viral load category on GAD-7 scores. The results indicated no significant main effect for age category, F (2, 1427) = 0.579, p = 0.560, partial η2 ; 0.001); no significant main effect for viral load category F([1, 1427) = 1.868, p = 0.172, partial η2 = 0.001); and no significant interaction age category and viral load category, F(2, 1427) = 0.151, p = 0.860, partial η2 = 0.001). ANOVA: Analysis of variance, GAD-7: Generalized anxiety disorder 7-item, η2: Eta squared - effect size measure.
Interactions between Age, Sex, VL, and PHQ-9 Scores
There was no interaction between the effects of age category and sex on PHQ-9 scores, F (2, 1427) = 0.016, p = 0.948). Simple main effects analysis showed that neither age group (p = 0.064) nor sex (p = 0.160) had a statistically significant effect on PHQ-9 scores [Figure 4].
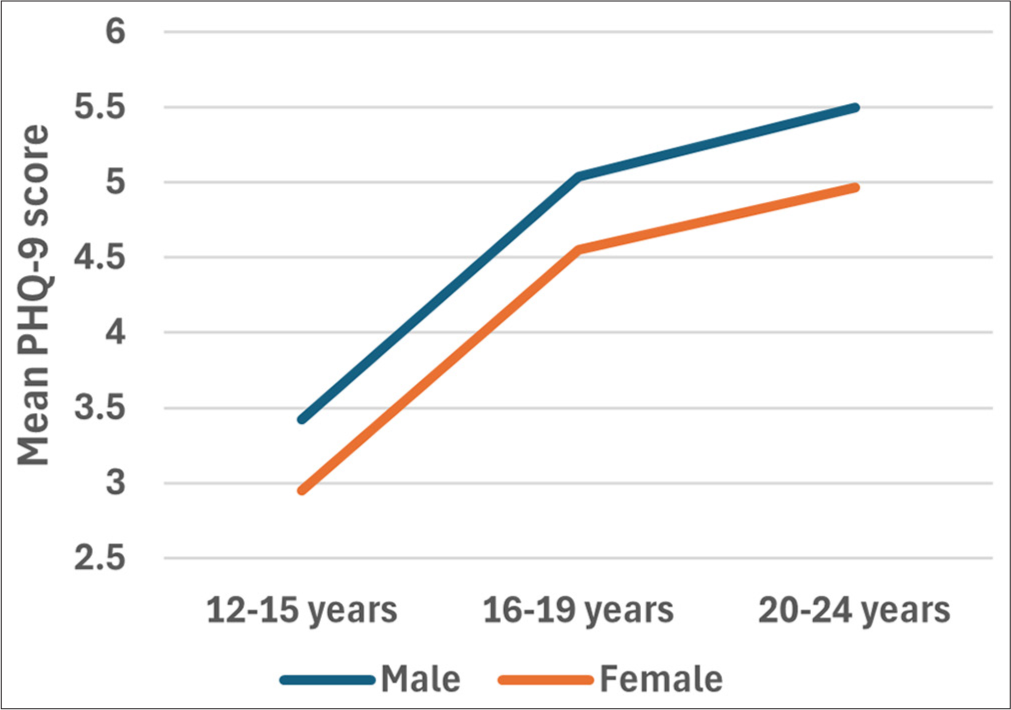
- Plots to display the mean PHQ-9 scores for different age categories based on their sex. A two-way ANOVA was performed to evaluate the effects of age group and sex l on PHQ-9 scores. The results indicated no significant main effect for age category, F (2, [1427) = 2.760, p = 0.064, partial η2 = 0.004; no significant main effect for sex, F (1, 1427) = 1.972, p = 0.160, partial η2 = 0.001; and no significant interaction between age category and sex, F (2, 1427) = 0.016, p = 0.984, partial η2 = 0.000 ANOVA, ANOVA: Analysis of variance, PHQ-9: Patient health questionnaire, η2: Eta squared -effect size measure.
There was no interaction between the effects of age category and VL category on PHQ-9 scores, F (2, 1427) = 0.148, p = 0.863). Simple main effects analysis showed that neither age group (p = 0.311) nor VL category (p = 0.479) had a statistically significant effect on PHQ-9 scores [Figure 5].
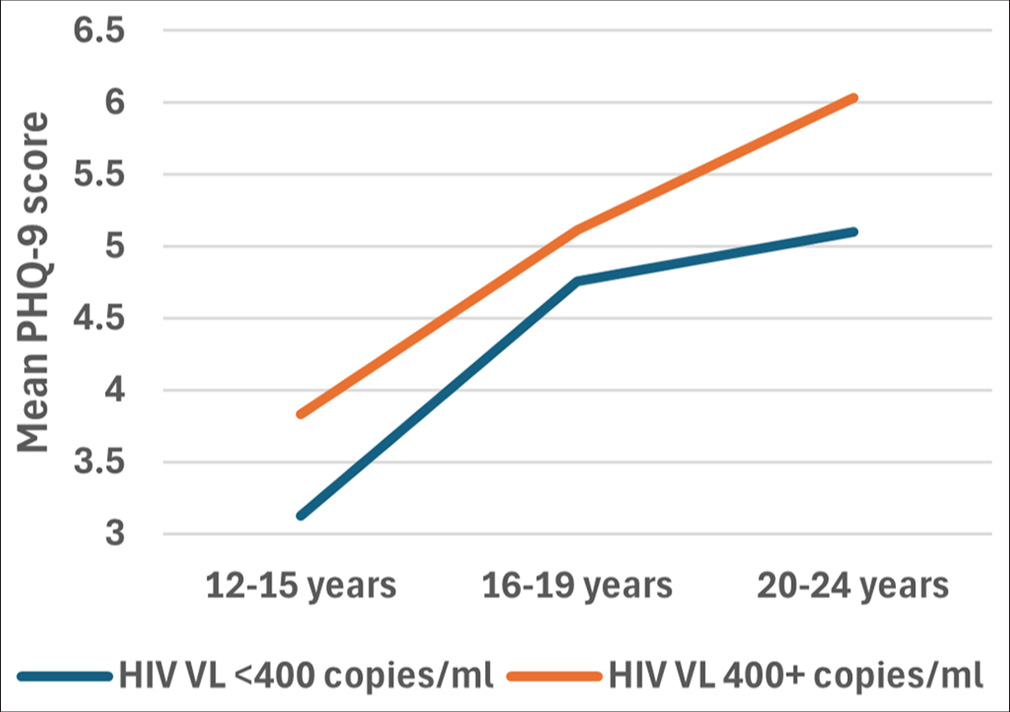
- Plots to display the mean PHQ-9 scores for different age categories based on their viral load (VL). A two-way ANOVA was performed to evaluate the effects of age category and viral load category on PHQ-9 scores. The results indicated no significant main effect for age category, F (2, 1427) = 1.170, p = 0.311, partial η2 = 0.002; no significant main effect for viral load category, F (1, 1427) = 0.502, p= 0.479 partial η2 = 0.000; and no significant interaction between age category and viral load category, F (2, 1427) = 0.148, p = 0.863, partial η2 = 0.000. ANOVA: Analysis of variance, PHQ-9: Patient health questionnaire, η2: Eta squared - effect size measure.
Sensitivity Analysis
When using HIV VL cutoff of <1000 copies/mL of blood, similar results were observed regarding HIV VTF rates (10%), the age distribution of participants by VTF status, the predictors of anxiety, depression, or both anxiety and depression symptoms, and the GAD-7 and PHQ-9 scores by VTF status. There were no interactions between the effects of age and sex or age and VL on GAD scores and PHQ-9 scores (Data not shown).
DISCUSSION
Results in Context
The age distribution of participants by VTF status in our study is consistent with prior literature that shows that those in the oldest age group were more likely to have VTF.[14-16] possibly due to the cognitive, emotional, and hormonal changes that older adolescents go through in the transition to adulthood[16] and the increased time to develop resistance to available medicines.[17]
However, HIV VTF rates (10%) were lower than those of similar populations in the same clinic (23.3% in 2012[18] and 17% in 2015[19]), potentially attributable to improved adherence and more robust treatment regimens resulting in viral suppression with less than perfect use that would override the effects of intermittent non-adherence that comes with the often-episodic nature of impairing anxiety or depression.[20] National data indicated that all AYAs were on a dolutegravir (DTG)-containing regimen by 2018, an integrase inhibitor (INSTI).[21] Furthermore, INSTI’s may change the strength of the association between VTF and mental health symptoms since they are more permissive of lower levels of adherence.[7,22] Therefore, patients on DTG with low levels of adherence who are virally suppressed would often not be referred for a mental health assessment.
Despite the absence of an association between either depression, anxiety, or both anxiety and depression and the likelihood of having VTF in this population, higher GAD-7 and PHQ-9 scores were observed among AYA with VTF that were not of statistical significance but are of clinical significance. In AYA populations globally, prior evidence of the relationship between mental disorders and HIV disease outcomes such as VTF is mixed.[23,24] Therefore, the relationship between mental illness and HIV treatment adherence is not straightforward. There may be several unmeasured confounders which can be explored in future studies. Furthermore, different HIV treatment regimens may modify the relationship between mental illness and treatment adherence, for instance, while DTG may decrease the likelihood of VTF, DTG increases the likelihood of symptoms of mental disorders.[25] Nonetheless, screening AYA with VTF for IPDs is useful to retain virological control and improve their health outcomes, especially where universal mental health screening of ART cohorts is not feasible.
The majority of patients with clinically relevant anxiety and depression (11% and 15%, respectively) did not have VTF, indicating that there is a substantial subset of patients who could benefit from referral for treatment of anxiety and depression symptoms regardless of their virologic outcomes. The fraction of AYA with clinically relevant symptoms shows that mental health screening should be more widespread among clinics treating HIV patients.[8] Moreover, older patients were more likely to have clinically relevant depression or higher GAD-7 scores, which may be related to the transitional phase where AYA must take on their own responsibilities, establish their independence, and face the consequences of their actions.[16] If universal screening is not feasible in a particular context, it may be useful to explore other measures of adherence, such as self-reports or pill counts targeted toward specific populations, like older AYA, to see if they show a greater ability to identify youth with symptoms of mental illness.
Study Strengths
Our study represents a year of mental health screening data in a high AYA-HIV burden country. Screening was self-administered on tablets to minimize social desirability bias. Moreover, to our knowledge, it is one of the first studies to examine the relationship between IPDs and HIV treatment adherence, using HIV VL as a proxy, facilitating the translation of our findings to treatment scenarios. Furthermore, similar results were obtained using HIV VL cutoff <1000 copies per ml of blood, illustrating the validity of our findings.
Study Limitations
First, HIV VL was only captured at a one-time point, which does not provide a longitudinal perspective on the course of treatment and the temporal association between VL assessment and symptoms of mental health disorders. However, HIV VL assessment was done within 6 months of mental health screening, an overlapping period. Moreover, previous studies in Botswana have shown that high scores on the Pediatric Symptom Checklist (PSC) were associated with recent HIV VL results, and baseline PSC scores predicted VTF in the next 6 months.[19]
Second, screening was done only once using self-administered screening (and not diagnostic) tools increasing the possibility of over or underreporting of symptoms. Moreover, clients with severe mental disorders may make fewer clinic visits and thus may not have been included in this cohort. Nevertheless, mental health screening was offered at least once per clinic visit over one year.[7] Patients with mild anxiety should be rescreened every 4 weeks to determine whether they warrant referral for medication[26] and be rescreened every 2–4 weeks and 3 months thereafter if on medication with follow-up schedules of 4–8 weekly.[27] We recommend that patients with clinically relevant categories be immediately referred for intervention, monitored within 2 weeks, and align their next follow-up appointments with their 3 monthly clinic visits.[7] Future studies could continuously evaluate the mental health and HIV virological levels of AYA such that each participant would serve as their own control.
Third, two-thirds of AYA in the clinic who were younger were ineligible for inclusion. We also have a long-standing cohort with more resources in an urban setting limiting the generalizability of study findings. Furthermore, the study was underpowered because only 11% of adolescents had VTF using a VL cutoff of <400 copies/ml, but we were still able to demonstrate a similar IPD burden between those with and without VTF. Future research should evaluate the relationship between VTF and mental well-being among larger populations of AYA of all age groups in diverse settings.
Fourth, because our secondary analysis was limited to the data collected for a separate study, we did not screen for substance abuse and alcoholism, which are likely to confound the relationship between symptoms of mental health disorders and VTF.[28] HIV is also commonly associated with social determinants of poor mental health, such as stigma, isolation, and parental infection,[29] and some ART regimens can have adverse neuropsychiatric effects.[30] In addition, limited HIV care information was available, such as the specific antiretroviral regimen or years of treatment, and this limited our ability to analyze the impact of these factors. However, we know that most participants were on DTG-based regimens, which is likely why we have a lower VTF rate than in other settings and older studies in the same setting. Future studies could incorporate more sociodemographic and clinical information and include more predictors that could confound the relationship between VTF and anxiety or depression symptoms.
CONCLUSION AND GLOBAL HEALTH IMPLICATIONS
HIV VTF may be a poor proxy measure to identify AYA with HIV who may have anxiety or depression symptoms. HIV programs should support universal screening among AYA for mental health comorbidities beyond what is done when clients have HIV VTF and promptly address mental health issues identified during screenings to improve health outcomes.
Key Messages
1) HIV virological treatment failure (VTF) may be a poor proxy measure to identify adolescents and young adults (AYA) with HIV who may have anxiety or depression symptoms. 2) HIV programs should support universal screening for mental health comorbidities beyond what is done when clients have HIV VTF. 3) Nonetheless, screening individuals with VTF on universal dolutegravir-based ART for mental health symptoms is useful to retain virological control and improve their health outcomes, especially where universal mental health screening of ART cohorts is not feasible.
Acknowledgments
The authors would like to thank Susan S. Lee and Kaja Darien for their editing of the final manuscript. We would also like to thank the patients, staff, and leadership of Botswana-Baylor Children’s Clinical Center of Excellence for in-kind support for this project.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest: The authors declare no competing interests. Financial Disclosure: Nothing to declare. Funding/Support: This project was supported by funding from the International AIDS Society, CIPHER programme; Penn Center for AIDS Research (CFAR), Penn Center for AIDS research (P30 AI 045008; Collman), an NIH-funded programme and the PENN Mental Health and AIDS Research Center((P30 MH 09748: Metzger), an NIH-funded programme. Ohemaa B. Poku is supported by awards (R01MH069133, PI: C. Mellins) and (P30MH43520, PI: R. Remien). Evan L. Eschliman is supported by award (T32DA031099, PI: Hasin/Martins). Ethics Approval: This study was reviewed and approved by the Institutional Review Board of the University of Pennsylvania (Protocol #829783[PENN-IRB- 21 May 2010 as conditional approval pending review of protocol and consent forms inconsistencies and final approval on 29 June 2018]), the Botswana Baylor Childrens Center of Excellence ( 02 March 2018 ), and the Health Research and Development Committee (HRDC) of the Ministry of Health of Botswana ( 26 March 2018). The University of Pennsylvania’s approval was conditional upon obtaining local Botswana approvals. All approvals were obtained prior to the commencement of research activities ([29 July 2018]) and were closed ([Penn: 09 May 2022, HRDC 10 March 2023). The study was conducted in accordance with all applicable ethical guidelines and regulations governing research involving human participants. Sub study to Penn #: 831643, Approved: 15 Oct 2018, closed 21 Feb 2022). Declaration of Patient Consent: The authors certify that they have obtained all appropriate patient consent. Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation: The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI. Disclaimer: None. †Equal Contributions: These authors contributed equally to the work.
References
- Second Botswana youth risk behavioural and biological surveillance survey report. Ministry of Basic Education;. :1-144.
- [Google Scholar]
- Psychiatric disorders in adolescents living with HIV in Botswana. AIDS Res Ther. 2023;20(1):2.
- [CrossRef] [PubMed] [Google Scholar]
- Symptoms of depression, anxiety, and thoughts of suicide/self-injury in adolescents and young adults living with HIV in Botswana. AJAR. 2023;22(1):54-62.
- [CrossRef] [PubMed] [Google Scholar]
- Mental health, substance use and viral suppression in adolescents receiving ART at a paediatric HIV clinic in South Africa. J Int AIDS Soc. 2020;23(12):e25644.
- [CrossRef] [PubMed] [Google Scholar]
- Risky behaviours and their correlates among adolescents living with HIV in sub-Saharan Africa: A systematic review. Reprod Health. 2018;15(1):180.
- [CrossRef] [PubMed] [Google Scholar]
- Mental health legislation in Botswana. BJPsych Int. 2019;16(3):68-70.
- [CrossRef] [PubMed] [Google Scholar]
- Handbook of the Botswana 2016 integrated HIV clinical care guidelines Gaborone. 2016. Botswana: The Ministry. [cited 2024 Aug 19]. Available from: https://www.childrenandaids.org/sites/default/files/2017-04/botswana_integrated-hiv-clinical-care-guidelines_2016.pdf
- [Google Scholar]
- Feasibility of integrating mental health screening into youth HIV Care in a Low-Middle Income Country Setting. Advance [A SAGE Preprints Community Preprint] 2021 Sep 10
- [CrossRef] [Google Scholar]
- Older adolescent (15 to 19 years) and young adult (20 to 24 years) mortality. 2022. [updated 2022 July 01; cited 2024 Aug 19] Available from: https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-older-adolescent-%2815-to-19-years%29-and-young-adult-%2820-to-24-years%29-mortality
- [Google Scholar]
- Considerations for statistical analysis In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a protocol for observational comparative effectiveness research. A user's guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013.
- [Google Scholar]
- A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med Res Methodol. 2013;13(1):92.
- [CrossRef] [PubMed] [Google Scholar]
- Botswana country operational plan 2019 strategic direction summary. 2019 May 10 [Updated 2023 Oct 01; cited 2024 Aug 19]. Available from: https://www.state.gov/wp-content/uploads/2019/09/botswana_cop19-strategic-directional-summary_public.pdf
- [Google Scholar]
- Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum. 2013;29(6):892-900.
- [CrossRef] [PubMed] [Google Scholar]
- Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One. 2012;7(12):e52856.
- [CrossRef] [PubMed] [Google Scholar]
- Virological failure among adolescents on ART, Harare City, 2017-a case-control study. BMC Infect Dis. 2018;18:469.
- [CrossRef] [PubMed] [Google Scholar]
- Rate of virological failure and HIV-1 drug resistance among HIV-infected adolescents in routine follow-up on health facilities in Cameroon. PLoS One. 2022;17(10):e0276730.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid psychosocial function screening test identified treatment failure in HIV+ African youth. AIDS Care. 2012;24(6):722-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of HIV virologic failure among adolescents using the pediatric symptom checklist. AIDS Behav. 2015;19:2044-8.
- [CrossRef] [PubMed] [Google Scholar]
- Adherence to and Forgiveness of 3TC/DTG in a Real-World Cohort. J Int Assoc Provid AIDS Care. 2022;21:23259582221101815.
- [CrossRef] [PubMed] [Google Scholar]
- Realizing the promise of dolutegravir in effectively treating children and adolescents living with HIV in real-world settings in 6 countries in Eastern and Southern Africa. Pediatr Infect Dis J. 2023;42(7):576-81.
- [CrossRef] [PubMed] [Google Scholar]
- Sexual transmission of HIV according to viral load and antiretroviral therapy: Systematic review and meta-analysis. AIDS. 2009;23(11):1397-404.
- [CrossRef] [PubMed] [Google Scholar]
- Psychiatric disorders, antiretroviral medication adherence and viremia in a cohort of perinatally HIV-infected adolescents and young adults. Pediatr Infect Dis J. 2018;37(7):673-7.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of mental health problems in adolescents living with HIV in Namibia. Child Adolesc Ment Health. 2017;22:179-85.
- [CrossRef] [PubMed] [Google Scholar]
- Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr. 2017;74(4):423-31.
- [CrossRef] [PubMed] [Google Scholar]
- Administration and scoring of the generalized anxiety disorder-7 (GAD-7) [technical supplement] 2020. PAR. [cited 2024 Aug 19]. Available from: https://www.parinc.com/portals/0/webuploads/samplerpts/checkit_series_gad7_tech_supp_paper_v4_092920.pdf
- [Google Scholar]
- Generalised anxiety disorder. 2024. [cited 2024 Aug 19]. Available from: https://patient.info/doctor/generalised-anxiety-disorder-pro#ref-11
- [Google Scholar]
- Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(2):159-66.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc. 2013;16(1):18593.
- [CrossRef] [PubMed] [Google Scholar]
- Neurotoxicity in the Post-HAART era: Caution for the antiretroviral therapeutics. Neurotox Res. 2016;30(4):677-97.
- [CrossRef] [PubMed] [Google Scholar]





