Translate this page into:
A Systematic Review of Interventions to Reduce Maternal Mortality among HIV-Infected Pregnant and Postpartum Women
✉Corresponding author email: SKonopka@msh.org
Abstract
Background:
In high-prevalence populations, HIV-related maternal mortality is high with increased mortality found among HIV-infected pregnant and postpartum women compared to their uninfected peers. The scale-up of HIV-related treatment options and broader reach of programming for HIV-infected pregnant and postpartum women is likely to have decreased maternal mortality. This systematic review synthesized evidence on interventions that have directly reduced mortality among this population.
Methods:
Studies published between January 1,2003 and November 30, 2014 were searched using PubMed. Of the 1,373 records screened, 19 were included in the analysis.
Results:
Interventions identified through the review include antiretroviral therapy (ART), micronutrients (multivitamins, vitamin A, and selenium), and antibiotics. ART during pregnancy was shown to reduce mortality. Timing of ART initiation, duration of treatment, HIV disease status, and ART discontinuation after pregnancy influence mortality reduction. Incident pregnancy in women already on ART for their health appears not to have adverse consequences for the mother. Multivitamin use was shown to reduce disease progression while other micronutrients and antibiotics had no beneficial effect on maternal mortality.
Conclusions:
ART was the only intervention identified that decreased death in HIV-infected pregnant and postpartum women. The findings support global trends in encouraging initiation of lifelong ART for all HIV-infected pregnant and breastfeeding women (Option B+), regardless of their CD4+ count, as an important step in ensuring appropriate care and treatment.
Global Health Implications:
Maternal mortality is a rare event that highlights challenges in measuring the impact of interventions on mortality. Developing effective patient-centered interventions to reduce maternal morbidity and mortality, as well as corresponding evaluation measures of their impact, requires further attention by policy makers, program managers, and researchers.
Keywords
Maternal mortality
HIV
Pregnancy
Postpartum
HIV-related interventions
Antiretroviral therapy
Systematic review
Background
Despite global progress in recent years, maternal mortality remains unacceptably high, particularly in developing countries.[1] In 2000, the global public health community agreed upon Millennium Development Goals (MDGs) and targeted a three quarters reduction in maternal mortality between 1990 and 2015. This target was not achieved, and with variable progress across regions and countries, there is an urgent need for improved understanding of the drivers of maternal mortality to inform a strengthened response.
In high prevalence populations, HIV-related maternal mortality is high, with increased mortality among HIV-infected pregnant and postpartum women compared to their uninfected peers.[2-4] A review of all causes of HIV-related morbidity and mortality in pregnant women from 1991 to 2009 estimated a seven- to eight-fold increased risk of pregnancy-related deaths in HIV-infected women compared to uninfected women.[5] In areas with low HIV-prevalence (less than 2 percent), 12 percent of pregnancy-related deaths are HIV-related, while in regions with high HIV-prevalence (greater than 15 percent), 50 percent of pregnancy-related deaths are HIV-related.[5]
The availability and incorporation of more effective treatment options such as antiretroviral therapy (ART) in country health programming is likely to decrease maternal mortality among HIV-infected pregnant and postpartum women. In an article about the South African process of confidential enquiries into maternal deaths, a reduction in deaths due to non-pregnancy related infections was described, the vast majority of which were among HIV-infected women.[2] These findings indicate that the scale-up of ART in pregnant women is beginning to have an effect on maternal mortality.[3] However, the evidence base is limited on ART's effect on maternal mortality, and even less is known about other interventions that may have an impact on reducing maternal mortality. This review was guided by the following question: Which interventions are beneficial in reducing maternal mortality in HIV-infected pregnant and postpartum women?
This review is one of three systematic reviews that collectively consider evidence on efforts to reduce mortality among HIV-infected pregnant and postpartum women. Two prior reviews examined the enablers and barriers to ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. The first review synthesized evidence on individual and contextual (or demand-side) factors,[6] while the second review examined the health system (or supply-side) barriers and enablers, as well as evidence on health system interventions that may facilitate access to ART by pregnant women.[7] This systematic review identifies interventions that prevent death among HIV-infected women during pregnancy and up to one year postpartum. Improved understanding, implementation, and scale-up of these will be critical to ensuring achievement of the new global targets for reductions in maternal mortality outlined in Sustainable Development Goal (SDG) 3.
Methods
Review Design
Following the PRISMA reporting framework, we conducted a systematic review to identify, analyze, and assess the effect of interventions that prevent death among HIV-infected women during pregnancy and up to one year postpartum.
Definitions
For our review, we define an intervention as a set of actions with a coherent objective to bring about change or produce identifiable outcomes.[8] We searched for interventions that were applied in clinical or public health settings. Public health interventions are intended to promote or protect health or prevent ill health in communities or populations. They are distinguished from clinical interventions, which are intended to prevent or treat illness in individuals.[8] ART is defined by the World Health Organization (WHO) as “treatment of people infected with human immunodeficiency virus (HIV) using anti-HIV drugs.” The standard treatment consists of a combination of at least three drugs (sometimes called “highly active antiretroviral therapy,” or HAART) that suppress HIV replication. Three drugs from at least two different drug classes are used in order to reduce the likelihood of the virus developing resistance. ART has the potential both to reduce mortality and morbidity rates among HIV-infected people, and to improve their quality of life.[9] Therefore, when this review references a study on ART, it refers to the triple therapy regimen described; when it describes studies using HIV therapies with fewer than three drugs, such as single dose nevirapine, we will refer to the medicine by its name.
Study Eligibility
Inclusion and exclusion criteria
The review included peer-reviewed studies published between January 1, 2003, and November 30, 2014, that reported interventions addressing mortality in HIV-infected pregnant or postpartum women. The timeframe was selected to maximize the relevance of the study findings to current clinical and public health policy and practice. Studies conducted in low-, middle-, and high-income countries were included if they were published in peer-reviewed journals and written in English. We excluded commentaries, letters, and viewpoints.
Search Strategy and Selection Process
Search strategy
Eligible peer-reviewed studies were identified by searching the PubMed database, a free search engine that comprises over 25 million citations from relevant biomedical literature and is widely used by the global health community. The search was conducted using a search term that combined three key elements: (1) HIV; (2) the population of interest (pregnant or postpartum women); and (3) the outcome of interest (mortality). We did not search gray literature such as websites of conferences, national ministries of health, or nongovernmental organizations.
Study selection
A multi-step strategy was followed to select studies. First, all studies not written in English were excluded. Then, records were excluded based on a title and abstract review. Next, two authors independently read and reviewed full-text articles, compared decisions about inclusion, and resolved discrepancies. Articles were excluded if they did not involve HIV- infected pregnant or postpartum women, did not describe an intervention, or did not include mortality as an endpoint. Figure 1 presents the PRISMA flow diagram for our review.
- PRISMA Flow Diagram. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting/terns for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal. pmed1000097
Data Extraction and Quality Assessment
Data extraction and management
A standard template was used for managing information extracted from included studies and was completed independently by two separate reviewers. Data extraction captured study objectives, setting, type, participants, sources of bias, and findings.
Quality assessment
We used the Quality Assessment Tool for Quantitative Studies developed by the Effective Public Health Practice Project to assess the quality and strength of included studies. This tool was selected because it allows for assessment of a variety of study designs and for studies whose data cannot be combined to produce a numerical synthesis, such as in a traditional meta-analysis.[10] We extracted eight key attributes of each study that relate to the quality and strength of the evidence. These include the following: (1) Selection bias; (2) Design; (3) Confounders; (4) Blinding; (5) Data Collection Methods; (6) Withdrawal and drop outs; (7) Intervention integrity; and (8) Analysis appropriate to question. Based on these eight characteristics, each study received a composite score of strong, moderate, or weak. The composite scores are reported in Table 1. Detailed assessment by attribute is presented in Table 2. No studies were excluded on the basis of the quality assessment. Rather, the quality assessment process was used to identify weaknesses in study methodologies and to strengthen interpretation and assessment of study findings.
| No. | Author | Publication year | Location | Study dates | Study design | Sample size | Study objective(s) | Quality of evidence |
|---|---|---|---|---|---|---|---|---|
| 1 | Aboud | 2009 | Malawi, Tanzania, Zambia | 2001-2003 | RCT | 1,829 | Metronidazole and erythromycin at 20-24 weeks or at delivery for three weeks | Moderate |
| 2 | Fawzi | 2007 | Tanzania | 1995-1997 | RCT | 1,078 | Daily Vitamin A, multivitamin or both | Moderate |
| 3 | Jamieson | 2007 | Malawi | 2004-2010 | RCT | 2,369 | Prevent MTCT | Strong |
| 4 | Kesho Bora | 2012 | Burkina Faso, Kenya and South Africa | 2005-2008 | RCT | 824 | Impact of postpartum ART discontinuation when used to prevent MTCT | Moderate |
| 5 | Kupka | 2008 | Tanzania | 2003-2005 | RCT | 815 | Daily selenium | Strong |
| 6 | Li | 2014 | Tanzania | 2004-2011 | Prospective | 18,917 | Risk factors for maternal mortality | Strong |
| 7 | Liotta | 2013 | Malawi, Mozambique | 2002-2010 | Retrospective | 10,150 | Pregnancy outcomes in women on ART, incident pregnancy in women on ART for their own health | Strong |
| 8 | Marazzi (a) | 2009 | Malawi, Mozambique | 2005-2007 | Prospective | 341 | Prevent MTCT | Strong |
| 9 | Marazzi (b) | 2011 | Malawi, Mozambique | 2005-2009 | Retrospective | 3,273 | Pregnancy outcomes in women on ART | Moderate |
| 10 | Matthews | 2013 | Uganda | 2005-2011 | Prospective | 354 | Incident pregnancy in women on ART for their own health | Strong |
| 11 | Melekhin | 2010 | USA | 1997-2008 | Retrospective | 193 | Impact of postpartum ART discontinuation when used to prevent MTCT | Strong |
| 12 | Nunn | 2011 | Zambia | 2000-2003 | RCT | 355 | Prophylactic cotrimoxazole | Moderate |
| 13 | Onen | 2008 | USA | 1997-2005 | Retrospective | 172 | Pregnancy outcomes in women on ART | Moderate |
| 14 | Shapiro | 2013 | Botswana | 2006-2008 | RCT | 730 | Impact of postpartum ART discontinuation when used to prevent MTCT, safety and efficacy of drugs | Moderate |
| 15 | Songok | 2003 | Kenya | 1996-1998 | Not described | 828 | Prevent MTCT | Weak |
| 16 | Thomas | 2011 | Kenya | 2003-2009 | Single arm trial | 522 | Safety and efficacy of drugs | Moderate |
| 17 | Tuomala | 2005 | USA | 1990-2002 | Prospective | 2,543 | Pregnancy outcomes in women on ART, safety and efficacy of drugs, incident pregnancy in women on ART for their own health | Moderate |
| 18 | Westreich | 2013 | South Africa | 2004-2011 | Prospective | 7,534 | Incident pregnancy in women on ART for their own health | Strong |
| 19 | Zvandasara | 2006 | Zimbabwe | 1997-2000 | RCT | 4,495 | Single, high-dose postpartum Vitamin A | Strong |
| No. | Author/ year | Selection bias | Design | Confounders | Blinding | Data collection methods | Withdrawal and drop outs | Intervention integrity | Analysis appropriate to question | Overall assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aboud (2009) | Participants are likely to be representative of the target population; 72% of screened individuals agreed to participate. MODERATE | Randomized Controlled Trial, double-blinded. Randomization described (separate publication). STRONG | There were important differences between groups (HIV status); this was accounted for in the design and analysis. STRONG | Double- blinded, placebo controlled. STRONG | Data collection tools were valid; Data collection tools were reliable. STRONG | Reason for loss to follow up provided (premature closure of study).WEAK | More than 80% of participants received the intervention of interest. Consistency of interventions was measured during frequent follow up visits. Unclear if participants received an unintended intervention. MODERATE | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; Can't tell whether the intervention allocation is based on intention to treat. MODERATE | MODERATE |
| 2 | Fawzi (2004) | Participants are likely to be representative of the target population; can't tell what percentage of screened individuals agreed to participate. MODERATE | Randomized controlled Trial - placebo -controlled, randomization method described. STRONG | Unlikely that there were major differences between groups prior to the intervention; baseline characteristics between intervention and placebo group similar. STRONG | Outcome assessors and participants unaware of intervention and exposure status. STRONG | Data collection described. Outcomes were reviewed and confirmed by study investigators. STRONG | Numbers lost to follow up not reported. Instead compliance to treatment reported. WEAK | 79% of participants received the intervention of interest for the duration of the study. MODERATE | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; Analysis based on intention to treat. STRONG | MODERATE |
| 3 | Jamieson (2012) | Participants are likely to be representative of the target population; 79% of screened individuals agreed to participate. MODERATE | RCT; Study described as randomized; Randomization method described; Randomization method appropriate. STRONG | No important differences between groups prior to interventions; Most confounders were controlled. STRONG | Outcome assessors unaware of intervention and exposure status; Can't tell whether study participants were aware of the study question. MODERATE | Data collection tools valid; Data collection tools were reliable; Internal checks in place to ensure consistency of data. STRONG | Reasons for loss to follow up not described. 23% loss to follow up at 24 weeks; By 48 weeks - 20% loss to follow up in the maternal ART group; 20% in the infant nevirapine group and 19% in the control group. MODERATE | More than 80% of participants received the intervention of interest. The consistency of the interventions was measured during frequent follow-up visits. Can't tell whether participants received an unintended intervention. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; Can't tell whether the intervention allocation is based on intention to treat. MODERATE | STRONG |
| 4 | Kesho Bora (2012) | Participants are likely to be representative of the target population; can't tell what percentage of screened individuals agreed to participate. MODERATE | Randomized Controlled Trial: Randomization method described. STRONG | No important differences between groups prior to interventions; Most confounders were controlled. STRONG | Outcome assessors and participants were not blinded. WEAK | Standardized case report forms used; data collection tools valid; Data collection tools were reliable; Internal checks in place to ensure consistency of data. STRONG | Reasons for loss to follow up not described. Loss to follow up at 18 months after delivery were 92.1% and 89.0% in the triple ARV arm and AZT/ sdNVP arm, respectively; at 24 months they were 86.1% and 84.7%, respectively. STRONG | More than 80% of participants received the intervention of interest. The consistency of the interventions was measured during frequent follow-up visits. Can't tell whether participants received an unintended intervention. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; Analysis based on intention to treat. STRONG | MODERATE |
| 5 | Kupka (2008) | Participants are likely to be representative of the target population; can't tell what percentage of screened individuals agreed to participate. MODERATE | Randomized controlled trial, placebo and double-blinded; randomization described. STRONG. | Unlikely that there were major differences between groups prior to the intervention; baseline characteristics between intervention and placebo group similar; STRONG | Outcome assessors unaware of intervention and exposure status; Can't tell whether study participants were aware of the study question. MODERATE | Data collection described. Outcomes were reviewed and confirmed by study investigators. STRONG | Numbers lost to follow up reported; 22% lost to follow up in placebo group, 25% lost to follow-up in the intervention group (CD4, CD8.CD3). MODERATE | More than 80% of participants received the intervention of interest. The consistency of the interventions was measured during frequent follow-up visits. Can't tell whether participants received an unintended intervention. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; Analysis based on intention to treat. STRONG | STRONG |
| 6 | Li (2014) | Participants likely to be representative of target population; can't tell about agreement to participate. MODERATE | Prospective cohort study. Nonrandomized. MODERATE | Not applicable | Not applicable | Data extracted from clinical records; comprehensive data quality controls in place such as double data entry systems; data collection tools were valid; data collection tools were reliable. STRONG | Not applicable | Likely more than 80% of women received the allocated intervention. The consistency of the intervention was measured. Can't tell whether subjects received an unintended intervention. MODERATE | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; STRONG | STRONG |
| 7 | Liotta (2013) | Participants likely to be representative of target population; can't tell about agreement to participate. MODERATE | Retrospective observational cohort study. Non-randomized. MODERATE | Important confounders existed prior to the intervention; Confounders addressed during analysis. MODERATE | Not applicable | Data collected from electronic medical records in a controlled study setting. Data collection tools were valid; Data collection tools were reliable. STRONG | Reasons for loss to follow up not described. Pre-delivery loss to follow up was 9.8% in women initiating ART during prenatal care and 3.9% in women initiating ART for their own health. Post-delivery abandonment of care was 7.8% in women initiating ART during prenatal care and 5.4% in women initiating ART for their own health. STRONG | Likely more than 80% of women received the allocated intervention. The consistency of the intervention was measured. Unlikely that subjects received an unintended intervention. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate. STRONG | STRONG |
| 8 | Marazzi (2009) | Participants likely to be representative of target population; can't tell about agreement to participate. MODERATE | Prospective observational cohort (Mother infant pairs); no control group. MODERATE | Not applicable | Not applicable | Data collected from electronic medical records in a controlled study setting. Data collection tools were valid; Data collection tools were reliable. STRONG | Reasons for loss to follow up not provided. 8% of pairs at 6 months and 17% of pairs at 12 months were lost to follow up. STRONG | Likely more than 80% of women received the allocated intervention. The consistency of the intervention was measured. Unlikely that subjects received an unintended intervention. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate. STRONG | STRONG |
| 9 | Marazzi (2011) | Participants likely to be representative of target population; can't tell about agreement to participate. MODERATE | Retrospective observational cohort. MODERATE | The control group (68 persons who did not receive ART) may be significantly different from others who did receive ART. Reasons for non-provis ion of ART not disclosed - however enrollment was based on the provision of ART to all participants. WEAK | Not applicable | Data collected from electronic medical records in a controlled study setting. Data collection tools were valid; Data collection tools were reliable. STRONG | Not applicable | Likely more than 80% of women received the allocated intervention. The consistency of the intervention was measured. Unlikely that subjects received an unintended intervention. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; STRONG. | MODERATE |
| 10 | Matthews | Participants likely to be representative of target population; can't tell about agreement to participate. MODERATE | Prospective cohort. No randomization. MODERATE | It is unlikely that there were major differences between the two comparison groups (baseline characteristics were compared); important confounders such as age, CD4 cell count, viral load, and number of children were incorporated. STRONG | Not applicable | Study occurred in a controlled setting. Data most likely extracted from medical records. Data validity and reliability not described. MODERATE | Reasons for loss to follow up not described; loss to follow up was low (3 and 7% at years 1 and 5 and did not differ among women with and without pregnancy (7 vs 8%). STRONG | All participant received the appropriate intervention; the consistency of intervention was measured (and steps taken to address loss to follow up); can't tell whether unintended interventions were received. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate. STRONG | STRONG |
| 11 | Melekhin (2010) | Participants likely to be representative of target population; can't tell about agreement to participate. MODERATE | Retrospective cohort; no randomization. MODERATE | No important differences between groups prior to the intervention; Groups were compared through baseline characteristics and outcome characteristics. STRONG | Not applicable | Data collection described. Outcomes were reviewed and confirmed by study investigators. STRONG | Withdrawals and drop outs not discussed with the exception of median follow up. 8% loss to follow up in the discontinuation group. STRONG | All participant received the appropriate intervention; the consistency of intervention was measured (and steps taken to address loss to follow up);can't tell whether unintended interventions were received. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate. STRONG | STRONG |
| 12 | Nunn (2011) | Yes - 1; Agreement to participate - 64%. MODERATE | Double blind randomized placebo controlled trial; Method of randomization described; Method appropriate. STRONG | No important differences between groups. STRONG | Outcome assessors unaware of intervention and exposure status; Can't tell whether study participants were aware of the study question. MODERATE | Data collection tools were valid; Data collection tools were reliable. STRONG | 44% of randomized women lost to follow up. Reasons for loss to follow up provided. WEAK | High level of loss to follow up - 44%; Regular follow up visits for participants during the study. Can't tell whether subjects received an unintended intervention. MODERATE to WEAK based on high loss to follow up | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; Analysis based on intention to treat. STRONG | MODERATE |
| 13 | Oneri (2008) | Participants likely to be representative of target population; can't tell about agreement to participate. MODERATE | Retrospective cohort. MODERATE | Can't tell whether there were confounders identified prior to the intervention; multivariate analysis conducted for potential confounders. MODERATE | Not applicable | Data extraction most likely done from medical records. Can't tell whether data collection tools were valid; Can't tell whether data collection tools were reliable. WEAK | Not applicable | All participants received the interventions as scheduled; consistency of the intervention was measured (efforts made to trace missing data); can't tell whether any unintended interventions were received. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate. STRONG | MODERATE |
| 14 | Shapiro (2013) | Participants are likely to be representative of the target population; 58% of screened individuals agreed to participate. MODERATE to WEAK | Randomized Controlled Trial; randomization process not described. STRONG | Likely there were important differences between groups (CD4 cell counts); this was accounted for in the design and analysis. STRONG | Blinding not described; no reference about the awareness of participants to the research question. MODERATE | Can't tell whether data collection tools were valid; Can't tell whether data collection tools were reliable. No reference to data management. WEAK | Reasons for loss to follow up not described. At 6 months less than 10% of participants were lost to follow up across all groups; at 24 months less than 80% of women had completed the full protocol in the randomization arms while more than 80% had completed the protocol in the control arm. However, vital status was known for almost 100% of participants across all arms. MODERATE | Almost 100% of participants received the allocated intervention; consistency of the interventions was measured; can't tell whether subjects received an unintended intervention. STRONG | Unit of allocation - individual; Unit of analysis - individual; statistical methods appropriate; Can't tell whether the intervention allocation is based on intention to treat. MODERATE | MODERATE |
| 15 | Songok (2003) | Yes - 1; Almost 100% of participants agreed to participate. STRONG | Method not stated. WEAK | Likely there were important differences between groups (44% did not accept intervention treatment); can't tell about control methods used for confounders. WEAK | Can't tell whether assessors were blinded to intervention; can't tell whether collection tools were shown to be reliable. MODERATE | No reference to data collection systems. Can't tell whether data collection tools were valid; Can't tell whether data collection tools were reliable. WEAK | Withdrawals reported with reasons for children; apparently 30 percent of children did not complete the study; loss to follow up for mothers not reported. WEAK | Less than 60% of participants received the intervention of interest; the consistency of the intervention was measured; can't tell whether the participants received an unintended intervention. WEAK | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; likely not an intention to treat analytic approach (surviving children with unknown HIV status excluded from the analysis). WEAK | WEAK |
| 16 | Thomas (201 1) | Participants are likely to be representative of the target population; 87% of screened individuals agreed to participate. STRONG | Open label, single arm clinical/ intervention trial. STRONG | Not applicable - no control group | Not applicable | Can't tell whether data collection tools were valid; Can't tell whether data collection tools were reliable. No reference to data management systems. WEAK | Reasons for loss to follow up partially provided; 20% of infants lost to follow up; 21 % of mothers were lost to follow up. MODERATE | 84% of participants adhered to treatment; consistency of the intervention was measured; can't tell whether the unintended interventions occurred. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; analysis based on intention to treat. STRONG | MODERATE |
| 17 | Tuomala (2005) | Can't tell whether participants are representative of the target population; Can't tell what percentage of individuals agreed to participate. WEAK | Prospective cohort. MODERATE | Important differences occurred in terms of the type of ART provided. Variables controlled by analysis. STRONG | Not applicable | Utilized medical records. Data quality checks in place. Data collection tools were valid; Data collection tools were reliable. STRONG | Not applicable | Likely more than 80% of women received the allocated intervention. The consistency of the intervention was monitored and changes in treatment regimens acknowledged. MODERATE | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; STRONG | MODERATE |
| 18 | Westreich (2013) | Participants likely to be representative of target population; likel) that the majority of participants agreed to participate. MODERATE | Prospective cohort. No randomization. MODERATE t f | Important differences between groups identified; potential confounders identified; most confounders controlled for in the analysis. STRONG | Not applicable | Used clinical records to extract data. Data collector tools were valid; Data collection tools were reliable. STRONG | Loss to follow up defined for data collection i and analysis purposes in the methods section. Not applicable | Likely more than 80% of women received the allocated intervention. The consistency of the intervention was monitored. MODERATE | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; STRONG | STRONG |
| 19 | Zvandasara (2006) | Participants likely to be representative of target population; Can't tell that the majority of participants agreed to participate. MODERATE | RCT. Randomization described. Method described. Method appropriate. STRONG | No important differences between groups. STRONG | Outcome assessors unaware of intervention and exposure status; Can't tell whether study participants were aware of the study question. MODERATE | Self-reported data by participants of utilization of health services. Serum values for Vitamin A and HIV tests were conducted at laboratories that participated in external qualit) assurance programs. Unable to comment on data collection tools for validity and reliability. MODERATE | Duration of follow up curtailed due to economic . reasons. 11% of HIV negative participants and 15% of HIV positive participants were lost to follow up at the end of 12 months. 84% f of HIV negative participants and 65% of HIV positive participants were lost to follow up at the end of 12 months. MODERATE | 100% of participants received the intervention of interest. The provision of interventions to participants is described. The administration of the intervention was once-off. STRONG | Unit of allocation - individual; Unit of analysis - individual; Statistical methods appropriate; Can't tell whether the intervention allocation is based on intention to treat. MODERATE | STRONG |
Results
Overview of Included Studies
We identified 1,373 records for review. We eliminated l,345 articles during the title and abstract review. We assessed 28 full-text articles for eligibility and excluded nine. See Figure 1 for details of the search.
Nineteen studies met the inclusion criteria for this systematic review. Fourteen were related to the provision of ART, while five addressed the effect of vitamins, micronutrients, and antibiotics on maternal and postpartum mortality. Detailed characteristics of the included studies are summarized in Table 1. We divided review findings into two categories: (1) findings related to ART interventions; and (2) findings related to non-ART interventions. In many cases, studies included important contextual information that requires consideration in the interpretation of their findings.
Findings Related to ART Interventions
Overview of ART studies
The 14 ART studies that met our inclusion criteria focused on a diverse group of study objectives and span a period of more than two decades. Studies assessed the effectiveness of ART in prevention of mother-to-child transmission (PMTCT) of HIV;[11-13] pregnancy outcomes in women on ART;[14-17] the clinical impact of postpartum ART discontinuation when used for PMTCT;[18-20] the impact of incident pregnancy in women on ART for their own health;[17,21,22] safety and efficacy of drugs, including comparisons of dual versus triple therapy regimens and nucleoside reverse transcriptase inhibitors versus protease inhibitor-based ART;[16,20,23] and risk factors for maternal mortality.[24]
Study designs included nine cohort studies (four retrospective[14,15,17,18] and five prospective [12,16,21,22,24]), three randomized control trials, (RCTs)[13,19,20] one single arm trial,[23] and one not stated.[11] Three studies were conducted in the United States,[14,16,18] and eleven were conducted in one or more countries in sub-Saharan Africa.[11-13,15,17,19-24]
The timing of ART initiation varied according to study objectives and design. Women started ART at different times during the pre-pregnancy, pregnancy, and the intrapartum periods. One study reported initiation of ART exclusively prior to pregnancy;[21] four prior to or during pregnancy;[14,17,22,24] three before the end of the second trimester;[12,15,20] and four studies during the third trimester, including the intrapartum period.[11,13,19,23] Timing of ART initiation during pregnancy is unspecified in two instances[14,18] and occurred anytime during pregnancy in one study.[16] Figure 2 depicts timing of ART by study, in detail.
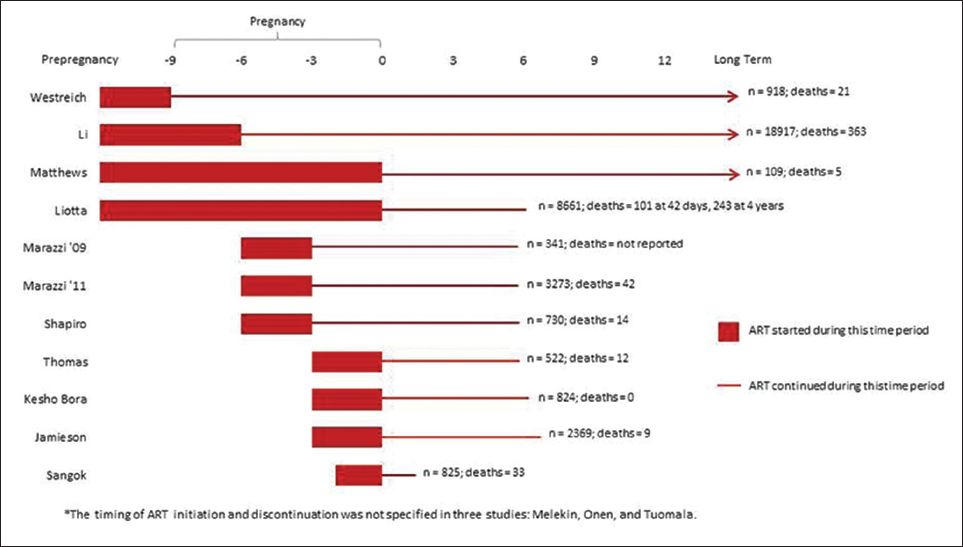
- Timing of ART Initiation and Duration of ART Provision by Study*
Similarly, ART cessation and long-term continuation varied between studies, according to study objectives. In two studies that focused on PMTCT, ART was provided for the duration of breastfeeding: 24[12] or 28 weeks postpartum.[13] In one instance, single dose-nevirapine was provided during the intrapartum period only.[11] In the studies that aimed to measure outcomes of HIV-infected women on ART, postpartum ART discontinuation occurred at four weeks postpartum[19] (nonbreastfeeding arm), 24 weeks,[15,20,23] or 26 weeks[19] (breast feeding arm). Four studies describe the provision of long-term ART for the mothers’ own health.[21,22,24] In one study, women taking ART for PMTCT stopped at 26 weeks and those who needed ART for their own health took it long term.[17] The timing of ART discontinuation was not specified in three studies.[14,16,18]
Where documented, the duration of the tracking of death as an outcome varied. Three studies tracked mortality to 42 days postpartum based on the standard definition for the maternal mortality ratio.[15,17,24] Three studies tracked outcomes to 48 weeks or one year postpartum,[12,13,22] four studies for 24 months postpartum,[11,19,20,23] one study up to four years post-delivery,[17] and three for the duration of the study.[14,18,21]
Key findings
ART provided during pregnancy reduces maternal mortality
Of the four studies that analyzed the relationship between ART and maternal mortality, all found that ART reduces maternal mortality.[15,17,22,24] One study was able to estimate a 34% decreased risk of maternal mortality among women on ART at delivery compared to those not on ART.[24]
ART's impact on maternal mortality is modified by HIV disease stage and CD4+ count
Certain studies measured women's CD4+ counts or assessed their HIV disease stage to determine whether ART affects the risk of maternal mortality at different CD4 counts or disease stages. A Ugandan study suggests that there is an increased risk of mortality in the pregnancy and postpartum period among HIV-infected women initiating ART at advanced disease stages, particularly during early ART.[22] This finding is supported by additional studies.[15,17,24] One study found that ART reduces maternal mortality at all CD4+ levels with at least a two-fold greater reduction in mortality among women with CD4+ counts above 350 cells/mm3 compared to those below 350 cells/mm3.[15]
Longer duration of ART during pregnancy is associated with a reduction in maternal mortality
Four studies found a reduction in maternal mortality associated with longer duration of ART.[15,17,22,24] Marazzi found a 70% reduction in the risk of maternal mortality among women who received more than 30 days of ART prior to delivery compared to those with less than 30 days.[15] Liotta found the shortterm mortality of women taking ART for less than 30 days was 2.2% compared to those receiving 31 to 90 days of ART (1.1%) and 91 to 270 days of ART (0.6%).[17] In the same study, maternal mortality was higher with shorter antenatal ART, regardless of w9hether the deaths took place over shorter (< 42 days post-delivery) or longer term (up to four years post-delivery).[17] More specifically, with respect to long-term mortality (up to four years post-delivery), mortality of women who received less than a month of ART was over two times higher (4.5%) than women who received three or more months of ART (1.8%; p<0.0001).[17] Li estimated that initiating ART before pregnancy was associated with a 55% decreased risk of maternal mortality, and the risk of mortality decreases by 8% for each additional month on ART.[24]
Discontinuation of ART for PMTCT leads to disease progression and mortality
Two studies reported on disease progression (including maternal mortality) after discontinuation of ART for PMTCT. In the study by Shapiro, most deaths in the randomized groups occurred after stopping ART.[20] Eight deaths occurred during six to 24 months postpartum (1.0/100 person years), whereas only one death occurred between ART initiation and cessation at six months postpartum.[20] Furthermore, among the eight deaths that occurred after ART cessation, three had started taking ART again but five had not. The authors calculated an absolute mortality risk of 1.4% in the 18 months following ART cessation, though not statistically significant.
The Kesho Bora study aimed to determine whether stopping ART once the infant is weaned affects 18 to 24 month maternal HIV disease progression.[19] In this study, progression was defined as WHO clinical stage 4, CD4+ count less than 200 cells/mm3, or death.[19] Postpartum HIV disease progression risk after ART discontinuation was 15.0% (two deaths, two with WHO stage 4 disease and 39 with at least one CD4+ count less than 200mm3). HIV disease progression varied by CD4+ count. Among women with counts of 200 to 349 cells/mm3 at enrollment, 24.0% (95% confidence interval [CI], 15.7-35.5) were found to have progressed. At counts above 350 cells/mm3, 4.9% (95% CI, 1.6-14.5) progressed.
Incident pregnancy in women on ART for their own health does not have adverse consequences for the mother
Four studies commented on incident pregnancies,[14,17,21,24] that is pregnancies that occurred in a cohort of women taking ART for their own health. Westreich found that incident pregnancy was not associated with an increased hazard and risk of death.[21] Li estimated that women initiated on ART before pregnancy had a statistically significant 55% decreased risk of maternal mortality.[24] And Liotta found that mortality within 42 days of delivery was lower among women who started ART prior to pregnancy compared to those who started during pregnancy.[17]
Two studies described the relationship between incident pregnancy in women on ART and adherence to treatment and abandonment of care. Westreich found that incident pregnancy was associated with a reduced hazard of becoming lost to follow-up (HR=0.62, CI. 0.51, 0.75) compared to the population of women on ART (not pregnant).[21] Liotta found that loss to follow-up before delivery in the PMTCT group was 9.8% versus 3.9% in women on established ART (p<0.001); abandonment of care was more frequent in the PMTCT group (7.8%) as opposed to women on established ART (5.4%) (p<0.001).[17]
Findings Related to Non-ART Interventions
Overview of non-ART studies
Five included studies described the impact of interventions such as vitamins/micronutrients and antibiotics on maternal and postpartum mortality. All five studies were conducted in one or more countries in sub-Saharan Africa.
Key findings
Multivitamins, vitamin A, and selenium have a limited or no beneficial effect on maternal mortality
Two studies of vitamin A supplementation met the study inclusion criteria. A randomized controlled trial in urban Tanzania that included four arms (vitamin A, multivitamin without vitamin A, multivitamin plus vitamin A, and placebo) found that the women in the multivitamin group were less likely to progress to stage 4 disease or death compared to the women who received a placebo.[25] In the randomized control trial study conducted in urban Zimbabwe, a single dose of vitamin A given postpartum had no effect on maternal mortality.[26] A randomized controlled trial of selenium supplementation in antenatal clinics in urban Tanzania found that selenium supplements had no significant effect on maternal mortality.[27]
Antibiotics had no effect on maternal mortality
We found two antibiotics studies that met the study inclusion criteria. The randomized controlled trial in Malawi, Tanzania, and Zambia found that erythromycin, ampicillin, and metronidazole given during pregnancy had no effect on maternal mortality.[28] Likewise, in the double-blind placebo controlled trial in Zambia, cotrimoxazole given after childbirth did not reduce maternal mortality.[29]
Conclusion
Interventions for HIV-infected pregnant and postpartum women, identified through the systematic review, encompass the provision of ART, micronutrients (multivitamins, vitamin A, and selenium), and antibiotics. No studies were found for interventions targeted at reducing important causes of death among HIV-infected pregnant or postpartum women such as severe malaria and TB.[4,30] Nor were any interventions found that attempt to address maternal mortality through nontherapy based approaches, for example, the use of quality assurance processes that promote clinical or obstetric audit[30,31] or community level activities that promote adherence to care.[32]
The two-decade span of studies reflects longstanding interest in identifying interventions that address the problems faced by HIV-infected pregnant and postpartum women in an expanding epidemic prior to 2010. Most studies identified focused on ART and explore different aspects of the relationship between ART and pregnancy, including drug safety and efficacy, PMTCT, and ART for the mother's own health. In contrast to ART with a demonstrated effect on outcomes, other interventions showed limited effects in reducing maternal mortality, with the exception of multivitamins that were shown in one study to reduce progression to death or advanced disease.
The review provides greater understanding about the contribution of ART in reducing maternal mortality. There is evidence that ART provision to pregnant and postpartum women reduces mortality, with extended periods of ART administration (more than 30 days) being superior to short-term provision. Findings from the review suggest that in the immediate period after ART initiation (< 30 days) women are subject to a higher risk of mortality,[15,17,22,24] especially women with advanced disease. This insight suggests the need to implement mitigation strategies aimed at managing the increased risk during early ART treatment, such as delayed pregnancy in nonpregnant women or heightened clinical vigilance, intensive follow-up, and optimal use of preventive therapies such as Intermittent Preventive Therapy for malaria in pregnant women.[33]
The review highlights two contrasting approaches in ART provision related to pregnancy. The approach of discontinuing ART in the immediate postpartum period provides evidence that the health gains of ART are reduced once treatment ceases, with a resurgence of mortality (although not statistically significant) and disease progression and the need to re-initiate ART for the mother's own health. On the other hand, findings about incident pregnancy in women on ART are encouraging and suggest that there appears to be no reason to limit pregnancy in women receiving ART for their own health. This does not preclude key precautions to prevent viral transmission for discordant couples. Also, adherence to ART appears to be superior when compared to women who initiate ART during pregnancy. This suggests the importance of implementing recommended approaches that favor long-term provision of ART rather than interrupting ART after weaning. Current ART guidelines[33] that recommend initiation of lifelong ART for all pregnant and breastfeeding women living with HIV (for example Option B+), regardless of their CD4+ count, are an important step in ensuring that pregnant women receive appropriate care and treatment.
Based on findings from the literature review, the effect of ART in reducing maternal mortality may not predict outcomes under operational conditions. Firstly, research in two cohorts occurred in controlled study settings (Malawi/Mozambique and Uganda). Treatment and care in the cohorts included the use of electronic monitoring systems (Malawi/ Mozambique), community-level support and active follow-up during treatment (Malawi/Mozambique and Uganda), and access to real-time laboratory testing (Uganda) - service elements not typical of most African service delivery systems. Secondly, the Malawi/Mozambique cohort includes clients from multiple sites in the two countries. It is not clear whether general health system performance in these two low-resource countries may have contributed to maternal mortality in HIV-infected women either through inadequate provision of obstetric services or deficiencies of care for common opportunistic infections that occur in these settings. Additionally, in one study,[15] the mortality effect is determined by comparing t he effect of duration of ART with a group of study participants who were not treated with ART (five deaths/68 participants not on ART, 10 deaths/365 participants with less than 30 days of ART, and 17 deaths/1,470 participants with 31 to 90 days of ART). The authors do not describe the reasons for non-ART initiation and this may have contributed to a higher risk for mortality based on exclusion from treatment.
Maternal mortality is a rare event and authors[21,28] highlight challenges related to inadequate sample sizes and the inability to generate significant outcomes. A number of groups attempted to overcome this limitation by developing combined end-points that include death, progression to stage 4 AIDS, and a CD4+ count below 200 [lowered CD4+ count].[18,19,21] Others have suggested that alternative approaches be developed to track progress without the need to rely on mortality as a marker. Suggested approaches may include assessment of process measures, such as patterns of prenatal clinic attendance, to increase the ability to measure effects of ART.[30] Developing effective approaches to measure the impact of interventions aimed at reducing maternal morbidity and mortality requires further attention.
Limitations
Our review has some limitations. Maternal mortality remains a relatively rare event, even in the presence of an extensive HIV epidemic. Our focus on maternal mortality rather than maternal morbidity may have excluded potential interventions that impact mortality, especially when research was focused on morbidity outcomes. The review therefore does not address important questions highlighted in studies that have explored the relationship between ART and pre-eclampsia/eclampsia, TB, and malaria.[34-39]
Selected studies targeted a diverse set of objectives and study outcomes. The majority of studies did not include maternal mortality as a primary outcome, and in certain studies, mortality was included as part of the descriptive statistics without further analysis or comment.[11,13] The limited number of studies reduces the basis from which conclusions can be drawn. Since 2009, the number of HIV-infected pregnant women receiving ART for their own health has grown from 25% to 60% in 2012.[2] Most studies identified through the review were initiated and concluded prior to this rapid period of expansion. It is likely that research initiated during the period of expanded coverage of ART will generate more concrete evidence about the contribution of ART to reduced maternal mortality.
Finally, this review covered only English-language studies and those published in PubMed. It is possible that there are relevant studies published in languages other than English and/or published in other sources of biomedical literature.
Global Health Implications
This systematic review consolidated evidence that ART reduces mortality in HIV-infected pregnant and postpartum women. Similarly, it is clear that the few interventions other than ART that have been tested have shown limited evidence of impact while no evidence is provided for interventions that target important causes of mortality in HIV- infected women (TB, malaria, and sepsis). This suggests that as ART scale-up continues, there is a need to explore the contribution of interventions that act synergistically with and complement ART to produce optimal outcomes in HIV-infected pregnant and postpartum women. These should include interventions that target not only important causes of morbidity and mortality, but also interventions that address nutritional status, the use of family planning methods to delay pregnancy during the initial phase of ART initiation in non-pregnant women, and quality assurance approaches such as clinical and obstetric audit or mechanisms that support initiation and adherence to care. As the global public health community consolidates strategies to End Preventable Child and Maternal Deaths and bring about an AIDS-Free Generation, the scale up of ART should be integrated with other patient-centered interventions that can further decrease maternal morbidity and mortality in HIV-infected pregnant and postpartum women.
Ethical Review:
This study is based on the review of existing studies and biomedical database.
Acknowledgements:
We would like to thank Scott Kellerman (MSH) for his review and input into earlier drafts of this manuscript, Alison Corbacio (MSH) for her work on tables and figures, and the authors of the companion systematic reviews on HIV and maternal mortality for their contributions to the team. We would also like to thank Sylvia Alford, Marta Levitt, and Allisyn Moran at the United States Agency for International Development (USAID) for their input into earlier drafts and during the review process. This publication was produced by the African Strategies for Health project at Management Sciences for Health with funding from the Africa Bureau of USAID. The authors’ views expressed in this publication do not necessarily reflect the views of USAID or the US Government.
Conflict of Interest:
The authors declare that they have no conflicts of interest relevant to this study.
References
- Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva, Switzerland: World Health Organization; 2015. Contract No.: ISBN 978 92 4 156514 1
- [Google Scholar]
- 2013 Progress Report on the Global Plan: Towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. UNAIDS; 2013.
- [Google Scholar]
- Tenth interim report on Confidential. Enquiries into Maternal Deaths in South Africa 2011 and 2012. Department of Health, South Africa 2013
- [Google Scholar]
- An autopsy study of maternal mortality in Mozambique: The contribution of infectious diseases. PLoS Medicine. 2008;5(2):e44.
- [CrossRef] [PubMed] [Google Scholar]
- The contribution of HIV to pregnancy-related mortality: A systematic review and meta-analysis. AIDS. 2013;27(10):1631-9.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS One. 2014;9(11):e111421.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS One. 2014;9(10):e108150.
- [CrossRef] [PubMed] [Google Scholar]
- Criteria for evaluating evidence on public health interventions. Journal of Epidemiology and Community Health. 2002;56(2):119-27.
- [CrossRef] [PubMed] [Google Scholar]
- Antiretroviral therapy. 2015 Available from: http://www.who.int/topics/antiretroviral_therapy/en/
- [Google Scholar]
- The use of short- course zidovudine to prevent perinatal transmission of human immunodeficiency virus in rural Kenya. American Journal of Tropical Medicine and Hygiene. 2003;69(1):8-13.
- [CrossRef] [PubMed] [Google Scholar]
- Increased infant human immunodeficiency virus-type one free survival at one year of age in sub-saharan Africa with maternal use of highly active antiretroviral therapy during breast-feeding. The Pediatric Infectious Disease Journal. 2009;28(6):483-7.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal and infant antiretroviral regimens to prevent postnatal HIV-l transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379(9835):2449-58.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of postpartum HIV treatment discontinuation on long-term maternal outcome. Journal of the International Association of Physicians in AIDS Care (Chicago). 2008;7(5):245-51.
- [CrossRef] [PubMed] [Google Scholar]
- Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-l correlates with favorable pregnancy outcomes. AIDS. 2011;25(13):1611-8.
- [CrossRef] [PubMed] [Google Scholar]
- Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. Journal of Acquired Immune Deficiency Syndrome. 2005;38(4):449-73.
- [CrossRef] [PubMed] [Google Scholar]
- Reduction of maternal mortality with highly active antiretroviral therapy in a large cohort of HIV-infected pregnant women in Malawi and Mozambique. PLoS One. 2013;8(8):e71653.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum discontinuation of antiretroviral therapy and risk of maternal AIDS-defining events, non-AIDS-defining events, and mortality among a cohort of HIV-1 - infected women in the United States. AIDS Patient Care and STDs. 2010;24(5):279-86.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal HIV-l disease progression 18-24 months postdelivery according to antiretroviral prophylaxis regimen (triple-antiretroviral prophylaxis during pregnancy and breastfeeding vs zidovudine/single-dose nevirapine prophylaxis): The Kesho Bora randomized controlled trial. Clinical Infectious Diseases. 2012;55(3):449-60.
- [CrossRef] [PubMed] [Google Scholar]
- HIV transmission and 24-month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana. AIDS. 2013;27(12):1911-20.
- [CrossRef] [PubMed] [Google Scholar]
- Incident pregnancy and time to death or AIDS among HIV-positive women receiving antiretroviral therapy. PLoS One. 2013;8(3):e58117.
- [CrossRef] [PubMed] [Google Scholar]
- HIV-infected women on antiretroviral treatment have increased mortality during pregnant and postpartum periods. AIDS. 2013;27(Suppl 1):S105-12.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding-the Kisumu Breastfeeding Study, Kenya: A clinical trial. PLoS Medicine. 2011;8(3):e1001015.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal mortality among HIV-infected pregnant women in Tanzania. Acta Obstetricia et Gynecologica Scandinavica. 2014;93(5):463-8.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized trial of multivitamin supplements and HIV disease progression and mortality. New England Journal of Medicine. 2004;351(1):23-32.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality and morbidity among postpartum HIV-positive and HIV-negative women in Zimbabwe: Risk factors, causes, and impact of single-dose postpartum vitamin A supplementation. Journal of Acquired Immune Deficiency Syndrome. 2006;43(1):107-16.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: Effects on maternal and child outcomes. American Journal of Clinical Nutrition. 2008;87(6):1802-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of prenatal and perinatal antibiotics on maternal health in Malawi, Tanzania, and Zambia. International Journal of Gynaecology and Obstetrics. 2009;107(3):202-7.
- [CrossRef] [PubMed] [Google Scholar]
- Randomised, placebo-controlled trial to evaluate co-trimoxazole to reduce mortality and morbidity in HIV-infected post-natal women in Zambia (TOPAZ) Tropical Medicine and International Health. 2011;16(4):518-26.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of human immunodeficiency virus treatment on maternal mortality at a tertiary center in South Africa: A 5-year audit. Obstetrics and Gynecology. 2009;114(2 Pt 1):292-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reduction of severe acute maternal morbidity and maternal mortality in Thyolo District, Malawi: The impact of obstetric audit. PLoS One. 2011;6(6):e20776.
- [CrossRef] [PubMed] [Google Scholar]
- 2015 [4/9/15]. Available from: http://www.m2m.org/
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2013
- [Google Scholar]
- Risk of preeclampsia in HIV-positive pregnant women receiving HAART: A matched cohort study. Journal of Obstetrics and Gynaecology Canada. 2012;34(2):136-41.
- [CrossRef] [PubMed] [Google Scholar]
- Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS. 2006;20(1):59-66.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet. 2002;360(9340):1152-4.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of lopinavir/ ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. 2015;29(2):183-91.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: A cohort study. Lancet. 2002;359(9323):2059-64.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis in pregnant and postpartum women: Epidemiology, management, and research gaps. Clinical Infectious Diseases. 2012;55(11):1532-49.
- [CrossRef] [PubMed] [Google Scholar]





