Translate this page into:
Impact of a Maternal Prevention of Mother-to-child Transmission of HIV (PMTCT) Intervention on HIV-exposed Infants in Uganda
*Corresponding author email: reb336@drexel.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in this journal, is properly cited
Abstract
Background:
Uganda has successfully reduced pediatric HIV infections through prevention of mother-to-child transmission of HIV (PMTCT) programs, yet little is known about adherence to infant-specific components of interventions. We hypothesized that infants born to mothers receiving the WiseMama (WM) electronic drug monitoring (EDM)-based adherence intervention would have increased uptake of six-week post-natal nevirapine (NVP) infant prophylaxis and better adherence to six-week early infant diagnosis (EID) HIV testing.
Methods:
At two sites in Uganda, the Wise Infant Study (WIN) prospectively followed an infant cohort. Infants were born to women enrolled in an RCT testing the effect of real-time reminders delivered via EDM on maternal adherence to antiretroviral therapy. We assessed intrapartum and discharge receipt of NVP prophylaxis using pharmacy and infant HIV DNA testing laboratory data.
Results:
Of 121 women eligible for WIN, 97 (80%) consented and enrolled; 46 had been randomized to control and 51 to intervention. There were no differences in receipt of a six-week NVP supply (control 87%, intervention 82%, p = 0.53). Receipt of any NVP prophylaxis did not vary by delivery location (p = 0.35), and although 12% of infants were delivered at non-study health facilities, they were not less likely to receive NVP at discharge (p = 0.37). Among infants with a completed HIV test, there was no difference in mean time to first test (control 52 days (SD 18), intervention 51 days (SD 15), p = 0.86). Only one infant, in the control group, tested positive for HIV.
Conclusion and Global Health Implications:
We found no significant differences in adherence to infant PMTCT practices between intervention and control infants with relatively high rates of NVP receipt albeit with suboptimal adherence to six-week EID testing. Further work is needed to ensure improved access, uptake, and follow-up of HIV-exposed infants in the Option B+ era.
Keywords
Prevention of maternal to child transmission of HIV
HIV
Nevirapine
Antiretroviral therapy prophylaxis
Early infant diagnosis
HIV-exposed infants
1. Introduction
1.1. Background of the Study
In 2015, an estimated 1.8 million children under age 15 were living with HIV globally, of which 150,000 were newly infected.1 With adherence to appropriate prevention of mother-to-child transmission of HIV (PMTCT) practices, rates of vertical transmission to infants can be reduced from approximately 40% to less than 3%.2 The demonstrated efficacy of PMTCT remains dependent on both maternal and infant adherence to PMTCT activities. A wealth of research has described adherence to HIV medications in many populations across multiple settings, but less evidence exists regarding adherence to post-natal PMTCT activities such as antiretroviral therapy (ART) prophylaxis and infant testing for infants born to HIV-positive women.3-9
Uganda, where the prevalence of HIV in pregnant women is approximately 7%,10 has demonstrated success in PMTCT: in 2011, an estimated 27,660 infants were born infected with HIV and by 2015, perinatal infections decreased to 3,872, an 86% reduction.11 In 2013, Uganda adopted Option B+ for PMTCT, which provides for immediate initiation of ART for pregnant women for life, regardless of CD4 count, and mandates: a) daily nevirapine (NVP) prophylaxis for all HIV-exposed infants at birth, regardless of feeding modality, for six weeks; b) early infant diagnosis (EID) by HIV DNA testing at six weeks of life; and c) rapid initiation of ART for all infants who test HIV positive.11,12 Additionally, all infants with an initial negative HIV DNA test result at six weeks of age are retested at six months of age.11,12 Recent data from 2016 suggest that an estimated 97% of HIV-positive pregnant women in Uganda accessed ART, but only 38% of HIV-exposed infants received ART for PMTCT.11
1.2. Objectives of the Study
From June 2015 through January 2017, the WiseMama (WM) study implemented a randomized behavioral adherence intervention delivered by real-time enabled electronic drug monitors (EDM) among ART-naïve HIV-positive pregnant and postpartum women in Uganda compared to standard of care under Option B+.13 For this study, we conducted a nested sub-study of infants born to mother-subjects in the WM trial with the objective of better understanding pediatric retention in the PMTCT cascade and to assess whether the maternal feedback intervention improved pediatric adherence. Few studies to date have followed a cohort of infants through the PMTCT-cascade under current Option B+ conditions.
1.3. Specific Aims and Hypothesis
We hypothesized that infants born to mothers receiving the WM EDM intervention would have increased uptake of six-week post-natal NVP infant prophylaxis component of PMTCT and better adherence to six-week EID HIV testing.
2. Methods
2.1. Study Variables
This study was a mixed-methods prospective cohort study of infants born to women enrolled in the WM study in Uganda. Briefly, the WM study was a randomized controlled trial designed to evaluate the effect of real-time feedback based on EDM data on maternal ART adherence and retention among ART-naïve HIV-positive pregnant women.13 All WM participants used EDM devices to store their ART medication. Following enrollment and prior to randomization, participants used the EDM for a one-month lead-in period to ensure they could use the EDM without technical or behavioral barriers. The intervention spanned randomization to three months postpartum and consisted of a text message reminder if women failed to open their EDM device within 60 minutes of dose time, and monthly counseling using the previous month’s EDM adherence data if adherence was <95% in that month. Mothers in the control arm received standard of care and used the EDM device but did not receive text messages or data-informed counseling. WM study inclusion criteria are reported in Supplemental Digital Table 1; see Supplemental Digital Table 2 for WM baseline characteristics.
| Characteristic | Place of delivery | Control infants (n = 45) | Intervention infants (n= 50) | P-value | Total infants (n = 95) | Wilson confidence interval |
|---|---|---|---|---|---|---|
| Total deliveries* | Study health facility | 39 (87%) | 45 (90%) | 0.51 | 84/95 (88%) | 80.5-93.4 |
| Different health facility | 6 (13%) | 5 (10%) | 0.76 | |||
| Receipt of intrapartum nvp** | Study health facility | 31 (80%) | 35 (78%) | 0.99 | 66/83‡ (80%) | 69.6-86.8 |
| Different health facility | 4 (67%) | 3 (60%) | 1.00 | 7/11 (64%) | 35.4-84.8 | |
| Receipt of 6-week supply of nvp for infant** | Study health facility | 34 (87%) | 37 (82%) | 0.69 | 71/83‡ (86%) | 76.4-91.5 |
| Different health facility | 5 (83%) | 3 (60%) | 0.55 | 8/11 (73%) | 43.4-90.3 | |
Comparisons were made using
2.1.1. Study location and participants
Two public government health facilities in central Uganda that provide integrated antenatal and ART services served as study sites, one in an urban setting and one in a rural setting. All WM participants who had not had a fetal death or stillbirth prior to study recruitment were eligible for inclusion, which began in January 2016.
2.1.2. Data collection
Data on infant outcomes were obtained from health facility records using both maternal and infant names and clinic-administered medical record numbers; verification of infant NVP prophylaxis was corroborated with facility pharmacy logs where possible. Data collected included: date of birth, receipt of intrapartum NVP prophylaxis, receipt of a six-week supply of infant NVP prophylaxis on discharge, date of infant EID, and EID test result. Given the retrospective nature of the chart review, it was not possible to determine infant adherence to NVP prophylaxis; only receipt of NVP either at intrapartum at the health facility and/or on discharge could be measured. A single trained data collector extracted all data from health facility registers.
2.2. Statistical Analysis
Descriptive statistics and differences by WM randomization status in: 1) receipt of NVP intrapartum; 2) receipt of six-week supply of NVP on discharge; 3) time to EID testing; and 4) EID test results were generated and analyzed using two-sample tests of means and Pearson’s chi-squared tests using SAS Version 9.4 (The SAS Institute, Inc., Cary, NC). Infants were categorized into one of three groups depending on how many days elapsed from date of delivery to date of EID testing: 1) ≤ 42 days, 2) 43 – 49 days, or 3) > 49 days; infants could only be categorized once, and elapsed time was derived from health facility records. These categories were used to compare proportions of infants receiving EID in the control arm versus the intervention arm during certain time-intervals using Pearson’s chi-squared test. Lastly, time to EID testing was compared by randomization status using Kaplan-Meier analysis. To have statistical power to detect a 25% difference in proportion of HIV-exposed infants receiving NVP prophylaxis, and proportion receiving an HIV test at six weeks of age, between groups, we calculated that a sample size of 123 infants born to WM participants was required.
2.3. Ethical Approval
The Boston University Medical Campus Institutional Review Board and the Mildmay Uganda Research Ethics Committee approved the study. Research approval was also obtained from the Uganda National Council for Science and Technology. All enrolled women provided written informed consent prior to participation in WIN study procedures.
3. Results
3.1. Sociodemographic Characterisitcs Results
Of the total 133 WM study subjects, 121 were eligible for the WIN study. Of these, 97 (80%) consented and were enrolled. At the urban site (n = 46), 22 women enrolled in WIN had been randomized to the WM control arm and 24 to the WM intervention arm. At the rural site (n = 51), 24 women were randomized to the WM control arm and 27 to the WM intervention arm (Figure 1). Although there was a lag between initial WM recruitment (June 2015) and WIN enrollment (January 2016), during which time, WM women continued to be lost to follow-up (LTFU), there was no significant difference in mean number of days between WM and WIN enrollment time with respect to WM randomization status (control = 199 days (SD 64 days), intervention = 196 days (SD 61 days), p = 0.86); this held for both study sites. While women were expected to deliver at a WM study facility, some gave birth at a non-WM health facility (Figure 2). All women at the rural study site gave birth at a study heath facility, compared to only 76% of women at the urban site (Figure 2); however, there were no differences in delivery location at the urban site between control and intervention infants (Table 1).

- Retention of Wise Infant (WIN) participants. Of the 121 mother-infant dyads eligible for the WIN study, 97 dyads enrolled. LTFU = lost to follow-up; EID = early infant diagnosis of HIV
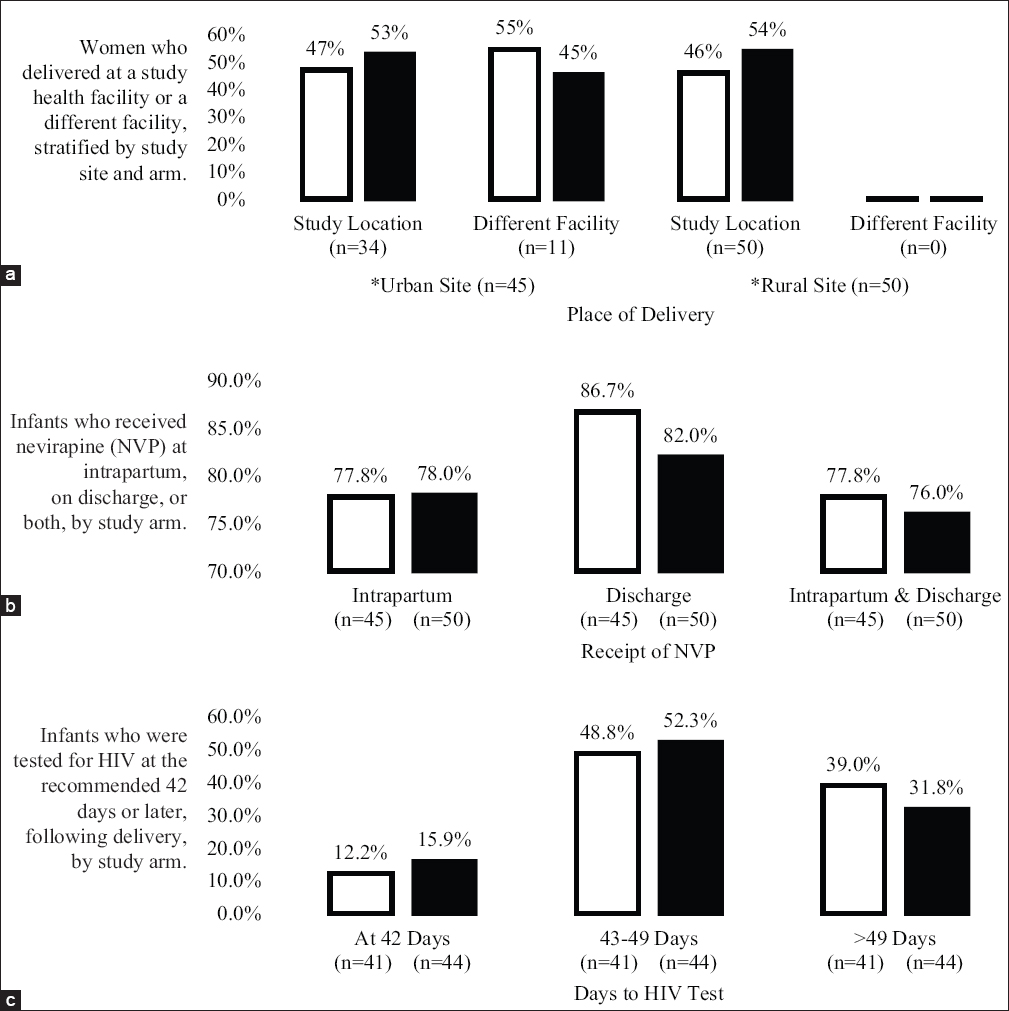
- Proportions of study participants and their respective place of delivery, receipt of NVP, and time to HIV testing. Legend: Intervention ◻ Control ◼
3.2. Receipt of NVP and Time to EID Testing
With respect to NVP prophylaxis, we found no differences in receipt of NVP prophylaxis between study arms: 78% of both control and intervention mother-infant dyads were recorded as receiving intrapartum NVP. There were also no differences in receipt of NVP prophylaxis for the first six weeks of life between study arms: 87% of control mother-infant dyads received NVP prophylaxis prior to discharge, and 82% of intervention mother-infant dyads received NVP (p = 0.53) (Figure 2). When stratified by study site, 86% of control dyads and 75% of intervention dyads received a six-week supply of NVP at the urban site (p = 0.46), and 87% of control infants and 88% of intervention infants received a supply at the rural site (p = 1.00). Although there were no significant differences in receipt of six-week NVP supply with respect to WM randomization status by study site, a slightly higher proportion of both the control and intervention mother-infant dyads received NVP at the rural study site compared to the urban study site.
Mean time to EID testing for study infants not LTFU was also similar between arms (control = 52 days (SD 18 days), intervention = 51 days (SD 15 days), p = 0.86). When stratified by study site, no difference between intervention and control infants was observed. At the urban site, mean time to EID testing was 52 days (SD 8 days) for the control group and 50 days (SD 12 days) for the intervention group (p = 0.67). At the rural site, the mean time to testing was 52 days for both the control (SD 24 days) and intervention (SD 18 days) groups (p = 0.99). Although not significant, infants took longer on average to present for EID testing at the rural site than at the urban site. Similarly, Kaplan-Meier analysis did not demonstrate a significant difference in median time to EID testing by study arm (control = 48 days, CI (46 – 55 days), intervention = 48 days, CI (45 – 53 days); log-rank p = 0.64; Figure 3). In analysis of proportion of infants receiving their EID test using three different time-interval categories in relation to their respective delivery date (i.e., ≤ 42 days, 43–49 days, and >49 days), there was no statistical difference in proportions of infants receiving EID testing stratified by WM randomization status among the categories (p=0.75) (Figure 2). Furthermore, when stratified by study site, proportions of infants in the three different categories did not differ statistically with respect to WM randomization status (urban site, p = 0.29; rural site, p = 0.70).
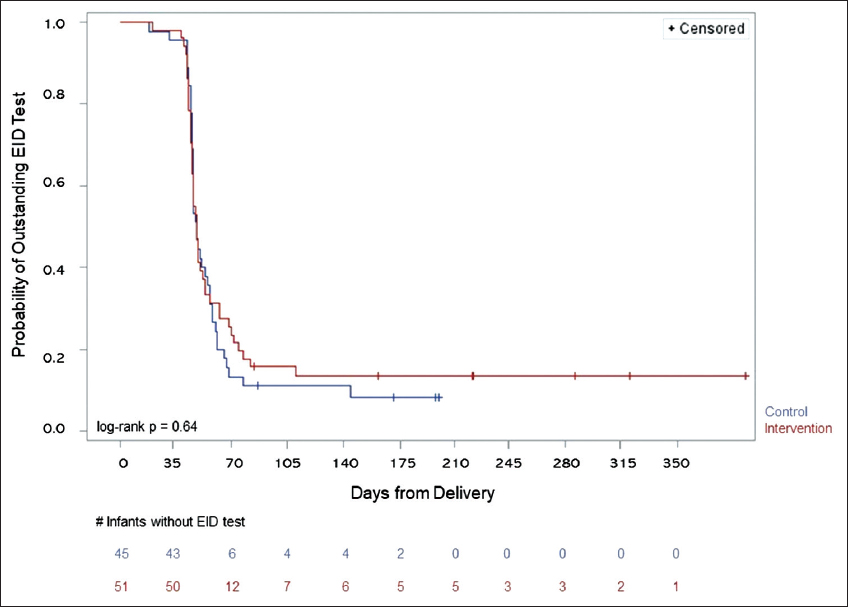
- Kaplan-Meier analysis of time to EID testing compared by randomization status. Given p-value is the significance between the infants in the control arm versus those in the intervention arm.
3.3. EID Testing Results and LTFU
Eleven infants were LTFU before EID testing (five control infants and six intervention infants; two at the urban site, nine at the rural site). One infant who was LTFU was reportedly kidnapped from the health facility prior to post-partum discharge; another, born to a woman in the intervention group, died at 30 days of life. Randomization status was not associated with infant LTFU status for any reason. Only one infant tested positive for HIV on EID. This infant was born to a woman who had been assigned to the control group. Forty-four days elapsed from delivery to EID testing, and this infant later died at 47 days of life (her HIV test returned positive after her death). One infant who was delivered at a study health facility did not have documentation regarding receipt of NVP prophylaxis. For another infant, the medical registry had information regarding receipt of NVP prophylaxis and time to EID testing for an infant, yet it did not have the infant’s place of delivery. These missing data points were non-differential between intervention and control groups.
4. Discussion
4.1. Discussion
This study is the first to our knowledge to report on infant access to the post-natal NVP prophylaxis components of PMTCT and completion of six-week EID HIV testing within the setting of a maternal ART adherence intervention, and is one of a limited number of studies that have followed a cohort of infants through the PMTCT-cascade under Option B+ conditions. While this study did not demonstrate a difference in NVP receipt for infant prophylaxis and time to EID testing with respect to the maternal EDM adherence intervention, it does illuminate trends of special concern within the infant PMTCT cascade.
With respect to our primary outcome of NVP receipt (both intrapartum and six-week supply), there was no significant difference between intervention and control mother-infant dyads. Per Option B+ policy, mother-infant dyads should receive NVP intrapartum, and all infants should get NVP prophylaxis for six-weeks postnatally at all public health facilities in Uganda. However, we found that any receipt of NVP among dyads was well below 100%. While all women delivered at the rural study site facility, only approximately half of women enrolled at the urban study site delivered at that study facility. It is critical that HIV-positive women deliver at a health facility that is aware of their HIV status, and ideally, the same facility at which antenatal care was received. Approximately 85% of exposed study infants received six-week NVP prophylaxis, which is much higher than 2016 national data reports for Uganda, with only 38% of exposed infants receiving prophylaxis.11 While high at 85%, our study’s reported receipt of NVP prophylaxis remains below the goal of 100% coverage of HIV-exposed infants, and is lower than Malawi, which reports upwards of 95% of exposed infants receiving prophylaxis.14,15
Over 85% of study infants, regardless of their mother’s randomization status, received EID testing within two months. Recent national data from Uganda report that only 33% of infants born to HIV-positive women received HIV testing within two months of birth in 2015.11 For the majority of study infants, the time to EID testing for both control and intervention arms was slightly greater than the recommended 42 days (median 48 days). While 48 days to EID is not markedly prolonged, diagnosis of HIV-positive infants as quickly as possible under current recommendations is crucial, given the early increased mortality risk for HIV-infected infants.
The WIN results are interpreted as intention-to-treat with respect to the WM study. Prior data suggest that initiating ART during pregnancy as opposed to during breastfeeding is a risk factor for poor retention in HIV care.16 In the WM study, ART adherence among all women was 77% over the entire intervention period, and it decreased significantly from the pre- to post-delivery period (68% versus 56%; p = 0.0029); this rate is far below the optimal 95% adherence threshold believed to best achieve long-term viral suppression.13,17,18 In WM post-intervention focus group discussions, participants stated that women are motivated to stay on ART during pregnancy to prevent HIV transmission to their babies (unpublished data).13 However, these women explained that high adherence was impeded by lack of HIV status disclosure to their male partners and by difficulty traveling to the clinic after delivery due to transportation cost, particularly if they had not disclosed their status to their partner (unpublished data).13 Given suboptimal ART adherence among pregnant women in the WM study, and that initiating ART during pregnancy has been identified as a risk factor for poor retention in HIV care, it is important that future interventions address the interpersonal, economic, and structural barriers to effective and early initiation of PMTCT.16,19
Although WHO does not currently recommend HIV DNA testing at birth for all exposed infants,12 as is done in some high-resource settings, the mortality outcomes and LTFU status that occurred in this study suggest a potential benefit to adding HIV DNA testing at birth to PMTCT programs in sub-Saharan Africa. In our study, one infant born to a mother in the control group tested positive for HIV at 44 days of life and later died at 47 days of life; another infant, born to a mother in the intervention group, died prior to EID testing at 30 days of life, with unknown HIV status. Eleven other infants in this cohort (11%) were LTFU before EID testing; while it is possible that they remained in care in other settings, they may have had delayed diagnostic testing, with potential for increased morbidity and mortality in this group.
4.2. Limitations
Study limitations included our inability to achieve the target sample size due to attrition in the WM study, resulting in a small sample size and limiting the power of this study. Further underpowering our ability to make inferences regarding primary outcomes were the additional infants LTFU throughout the study period. We observed differential retention between the urban and rural study sites, which is likely multifactorial, though this did not substantially affect WIN study recruitment. Incomplete routinely collected health facility data also affected the study, suggesting the general need for better surveillance to accurately capture health facility activity, such as PMTCT schedule completion. In order to surveil and evaluate PMTCT programs, complete records and documentation are essential.
5. Conclusion and Global Health Implications
Despite the limitations of this small study, the results confirm prior research regarding poor retention and health outcomes for HIV-exposed infants, both pre- and post-Option B+.11,20-23 All study infants had a mean time to EID testing that exceeded the recommended six weeks, and receipt of NVP prophylaxis was below intended targets. While multiple challenges and barriers impact the maternal component of PMTCT adherence, HIV-exposed infants are particularly vulnerable: infants depend on their mothers’ and other caregivers’ adherence to appropriate feeding modalities, administration of NVP prophylaxis, and scheduled EID testing. Although we could not demonstrate improved NVP receipt or infant retention for EID testing in the setting of a targeted maternal ART adherence intervention, these data support the urgent need for further investigation into barriers to and facilitators of PMTCT adherence among mother-infant dyads. Given the nearly one million pregnancies globally that are impacted by maternal HIV infection,10 with an estimated 2% affected by mother-to-child transmission even under optimal Option B+ scale-up and implementation, further delay in improving the care for these HIV-affected mother-infant dyads will continue to result in preventable deaths and other poor health outcomes.
Acknowledgements
We express our gratitude to the directors and staff of the hospital study sites and to Mildmay Uganda which supports the study sites through a partnership with the United States Centers for Disease Control and Prevention. We also thank the National Department of Health of Uganda for providing care for the patients. Above all, we thank the patients attending the antenatal clinics for their continued trust in the treatment provided.
Conflicts of Interest: No conflicts of interest for any authors to disclose.
Financial Disclosure: No financial disclosures for any authors to disclose.
Funding/Support: Funding was provided by an internal early career award to Rachael Bonawitz at the Boston University School of Public Health. Additional funding was provided by the United States National Institutes of Health, National Institute for Mental Health (NIH/NIMH 1R34MN103075). The contents are the responsibility of the authors and do not necessarily reflect the views of Boston University.
Ethics Approval: The Boston University Medical Campus Institutional Review Board and the Mildmay Uganda Research Ethics Committee approved the study. Research approval was also obtained from the Uganda National Council for Science and Technology.
Tribute: We are saddened to report that our co-author Ms. Chemusto passed away suddenly on May 26, 2020, after initial submission of this paper. She made significant contributions to the study design, execution and acquisition of data, as well as substantial critical review of this article. We acknowledge Ms. Chemusto’s contributions to this paper and dedicate this paper in her memory.
References
- 2016. For Every Child, End AIDS:Seventh Stocktaking Report, 2016. https://www.unicef.org/publications/
- Reducing the risk of mother-to-child human immunodeficiency virus transmission:past successes, current progress and challenges, and future directions. American Jounral of Obstetrics and Gynecology. 2007;197(3 Suppl):S3-9.
- [Google Scholar]
- Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS and Behaviour. 2010;14(3):580-589.
- [Google Scholar]
- Feasibility and Acceptability of a Real-Time Adherence Device among HIV-Positive IDU Patients in China. AIDS Research and Treatment. 2013;2013:957862.
- [Google Scholar]
- Improving Adherence to Antiretroviral Therapy With Triggered Real-time Text Message Reminders: The China Adherence Through Technology Study. Journal of Acquired Immune Deficiency Syndromes. 2015;69(5):551-559.
- [Google Scholar]
- The use of cell phone support for non-adherent HIV-infected youth and young adults: an initial randomized and controlled intervention trial. AIDS and Behavior. 2014;18(4):686-696.
- [Google Scholar]
- Improving adherence to antiretroviral therapy for youth living with HIV/AIDS:a pilot study using personalized, interactive, daily text message reminders. Journal of Medical Internet Research. 2012;14(2):e51.
- [Google Scholar]
- “+CLICK“: pilot of a web-based training program to enhance ART adherence among HIV-positive youth. AIDS Care. 2012;24(3):310-308.
- [Google Scholar]
- Feasibility of interactive text message response (ITR) as a novel, real-time measure of adherence to antiretroviral therapy for HIV+youth. AIDS and Behavior. 2013;17(6):2237-2243.
- [Google Scholar]
- AIDSinfo. http://aidsinfo.unaids.org/.
- The Uganda HIV and AIDS Country Progress Report July 2015-June 2016. https://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2017countries
- Programmatic Update:Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. 2012. Executive Summary. https://www.who.int/hiv/pub/mtct/en/
- [Google Scholar]
- Improving ART Retention and Adherence in Uganda:The Wise Mama Study (WiseMama). https://clinicaltrials.gov/ct2/show/NCT02396394
- HIV Self-Testing Africa Initiative Research. Government of Malawi. Ministry of Health. Integrated HIV Program Report January-March 2017. https://hivstar.lshtm.ac.uk/malawi-policies-strategies/
- [Google Scholar]
- HIV Self-Testing Africa Initiative Research. Government of Malawi. Ministry of Health. Integrated HIV Program Report April-June 2017. https://hivstar.lshtm.ac.uk/malawi-policies-strategies/
- [Google Scholar]
- Retention in HIV Care During Pregnancy and the Postpartum Period in the Option B+Era: Systematic Review and Meta-Analysis of Studies in Africa. Journal of Acquired Immune Deficiency Syndromes. 2018;77(5):427-438.
- [Google Scholar]
- Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21-30.
- [Google Scholar]
- Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33(8):1417-1423.
- [Google Scholar]
- Timing of pregnancy, postpartum risk of virologic failure and loss to follow-up among HIV-positive women. AIDS. 2017;17(31(11)):1593-1602.
- [Google Scholar]
- The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27(17):2787-2797.
- [Google Scholar]
- Identifying Gaps in Prevention of Mother to Child Transmission of HIV: A Case Series of HIV-positive Infants in Zambia. Pediatric Infectious Disease Journal. 2016;35(7):772-776.
- [Google Scholar]
- Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya. Tropical Medicine and International Health. 2010;15(7):833-841.
- [Google Scholar]
- Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20(8):958-968.
- [Google Scholar]
Supplemental Digital Content
| Inclusion criteria | Hiv+ pregnant women Between 18 and 59 years of age Receive antenatal care, are art-naïve, and initiate art at a study health facility Between 18 and 26 weeks of estimated gestation Be able to use a cell phone that can receive text messages Provide written informed consent |
| Exclusion criteria | Women with any previous experience with arv medications Women who cannot receive text messages in their respective homes Women who are unwilling to provide written informed consent |
| Characteristic | N (%) or Mean (SD) |
|---|---|
| Age (years) | 25.1 (5.6) |
| Married | 118 (74.2) |
| Education level completed | |
| Primary | 65 (40.6) |
| Secondary school | 83 (51.9) |
| First pregnancy | 44 (29.0) |
| Multiparous women, previous pregnancies | 2.5 (1.8) |
| Someone else knew HIV status at enrollment | 62 (38.8) |
| Disclosed HIV status to husband/partner at enrollment | 41 (26.1) |
| Completed pre-intervention period | 132 (80.0) |
| Adherence, pre-intervention period (%) | 76.0 (24.9) |





